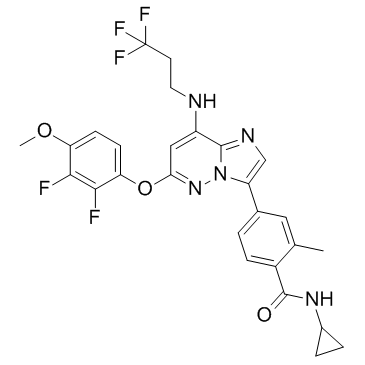
BAY 1217389 (BAY-1217389) is a novel, potent, orally bioavailable and selective inhibitor of the serine/threonine kinase monopolar spindle 1 (MPS1) (IC50 < 10 nM) with potential cancer activity by selectively binding to and inhibiting the activity of Mps1. This results in chromosomal misalignment and missegregation, accelerated mitosis, destabilization of the mitotic checkpoint complex, and inactivation of the spindle assembly checkpoint (SAC). In cancer cells that overexpress Mps1, this results in cell death.
Physicochemical Properties
| Molecular Formula | C27H24F5N5O3 |
| Molecular Weight | 561.5032 |
| Exact Mass | 561.179 |
| Elemental Analysis | C, 57.75; H, 4.31; F, 16.92; N, 12.47; O, 8.55 |
| CAS # | 1554458-53-5 |
| Related CAS # | 1554458-53-5 |
| PubChem CID | 78320750 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Index of Refraction | 1.617 |
| LogP | 4.64 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 40 |
| Complexity | 867 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | FC(C([H])([H])C([H])([H])N([H])C1=C([H])C(=NN2C1=NC([H])=C2C1C([H])=C([H])C(=C(C([H])([H])[H])C=1[H])C(N([H])C1([H])C([H])([H])C1([H])[H])=O)OC1C([H])=C([H])C(=C(C=1F)F)OC([H])([H])[H])(F)F |
| InChi Key | WNEILUNVMHVMPH-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C27H24F5N5O3/c1-14-11-15(3-6-17(14)26(38)35-16-4-5-16)19-13-34-25-18(33-10-9-27(30,31)32)12-22(36-37(19)25)40-21-8-7-20(39-2)23(28)24(21)29/h3,6-8,11-13,16,33H,4-5,9-10H2,1-2H3,(H,35,38) |
| Chemical Name | N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-(3,3,3-trifluoropropylamino)imidazo[1,2-b]pyridazin-3-yl]-2-methylbenzamide |
| Synonyms | BAY-1217389; BAY 1217389; 1554458-53-5; BAY1217,389; BAY-1217,389; Bay 1217,389; Benzamide, N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-[(3,3,3-trifluoropropyl)amino]imidazo[1,2-b]pyridazin-3-yl]-2-methyl-; M964LB1114; N-cyclopropyl-4-[6-(2,3-difluoro-4-methoxyphenoxy)-8-(3,3,3-trifluoropropylamino)imidazo[1,2-b]pyridazin-3-yl]-2-methylbenzamide; UNII-M964LB1114; BAY1217389 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Mps1/monopolar spindle 1 (IC50 = 0.63 nM) |
| ln Vitro |
BAY 1217389 has an IC50 value of 0.63±0.27 nmol/L in biochemical assays. The protein exhibits a high degree of selectivity towards other kinases and can bind to PDGFRβ (<10 nmol/L), Kit (between 10 and 100 nmol/L), CLK1, CLK2, CLK4, JNK1, JNK2, JNK3, LATS1, MAK, MAPKAP2, MERTK, p38β, PDGFRα, PIP5K1C, PRKD1, and RPS6KA5 (between 100 and 1,000 nmol/L). By causing an early exit from mitosis (referred to as "mitotic breakthrough") and thereby abrogating nocodazole-induced SAC activity, BAY1217389 induces multinuclearity and tumor cell death in cellular mechanistic assays. It has been discovered that it inhibits cell proliferation, with a median IC50 of 6.7 nmol/L (range: 3 to >300 nmol/L). BAY 1161909 and BAY1217389 inhibited Mps1 kinase activity with IC50 values below 10 nmol/L while showing an excellent selectivity profile. In cellular mechanistic assays, both Mps1 inhibitors abrogated nocodazole-induced SAC activity and induced premature exit from mitosis (“mitotic breakthrough”), resulting in multinuclearity and tumor cell death. Both compounds efficiently inhibited tumor cell proliferation in vitro (IC50 nmol/L range).[1] |
| ln Vivo |
BAY 1217389 exhibits a moderate level of efficacy when used in monotherapy in tumor xenograft studies. In the tested species, its blood clearance is found to be low. The terminal half-lives were long and the Vss was high. Male Wistar rats (0.5 mg/kg) and female NMRI mice (1 mg/kg) are given oral BAY 1217389. Peak plasma concentrations are measured in the 1.5–7 hour time range. In rats, oral bioavailability is high, whereas in mice, it is moderate[1]. In vivo, Empesertib (BAY1161909) and BAY1217389 achieved moderate efficacy in monotherapy in tumor xenograft studies. However, in line with its unique mode of action, when combined with paclitaxel, low doses of Mps1 inhibitor reduced paclitaxel-induced mitotic arrest by the weakening of SAC activity. As a result, combination therapy strongly improved efficacy over paclitaxel or Mps1 inhibitor monotreatment at the respective MTDs in a broad range of xenograft models, including those showing acquired or intrinsic paclitaxel resistance. Both Mps1 inhibitors showed good tolerability without adding toxicity to paclitaxel monotherapy. These preclinical findings validate the innovative concept of SAC abrogation for cancer therapy and justify clinical proof-of-concept studies evaluating the Mps1 inhibitors BAY 1161909 and BAY 1217389 in combination with antimitotic cancer drugs to enhance their efficacy and potentially overcome resistance [1]. |
| Enzyme Assay |
Through the phosphorylation of a biotinylated peptide (Biotin-Ahx-PWDPDDADITEILG-NH2), TRFRET-based in vitro kinase assays evaluate the inhibition of recombinant human Mps1 by BAY 1161909 or BAY 1217389. Standard assay conditions call for a 15-minute preincubation period between the kinase and test compound, followed by the addition of substrate and ATP at 10 μM to initiate the enzyme reaction.
Kinase assay [1] The inhibition of recombinant human Mps1 by Empesertib (BAY1161909) or BAY1217389 was assessed in TR-FRET–based in vitro kinase assays via phosphorylation of a biotinylated peptide (biotin-Ahx-PWDPDDADITEILG-NH2). Kinase and test compound were preincubated for 15 minutes before enzyme reaction was started by the addition of substrate and ATP upon 10 μmol/L. For further details, see Supplementary Materials and Methods. Kinase selectivity profiling [1] Empesertib (BAY1161909) and BAY1217389 were counterscreened against a panel of kinases using the Millipore Kinase or DiscoveRx profiler screen. Empesertib (BAY1161909) was initially tested at 1 μmol/L in the DiscoveRx kinase panel, followed by KD determination for 11 kinases (Supplementary Table S1). BAY 1161909 was tested at 10 μmol/L in the Millipore kinase panel, followed by retesting at 1 and 0.1 μmol/L and IC50 determination for JNK1alpha, JNK2alpha, and JNK3 (Supplementary Table S1). BAY 1217389 was initially tested at 1, 0.1, and 0.01 μmol/L in the DiscoveRx kinase panel (Supplementary Table S2). |
| Cell Assay |
Cells are seeded in the appropriate medium supplemented with 10% FCS into 96-well plates at densities ranging from 1,000 to 5,000 cells per well. Cells are treated with compound dilutions in quadruplicates after a 24-hour period. Adherent cells are stained with crystal violet and fixed with glutaraldehyde after an additional 96 hours. The company's software is used to calculate IC50 values through a 4-parameter fit. Multinuclearity assay. [2] U-2 OS (osteosarcoma ATCC: HTB-96) cells were plated at a density of 2,500 cells per well in a 384-well microtiter plate in 20 μL cell culture medium and incubated overnight at 37°C. Empesertib (BAY1161909) or BAY1217389 was added at a final concentration of 100 nmol/L in triplicates. Cells were treated for 0, 24, 48, and 72 hours at 37°C in the presence of test compounds. Thereafter, cells were fixed with 4% (v/v) PFA, permeabilized with 0.5% (v/v) Triton X-100, and blocked with 0.5% (v/v) BSA in PBS. α-Tubulin structures were detected by antibody labeling. After blocking with goat IgG, secondary antibodies were applied in the blocking solution. Cells were washed with PBS, and nuclei were marked with Hoechst 33342. Finally, cells were washed and covered with PBS and stored at 4°C until image acquisition. Images were acquired with a PerkinElmer Opera High-Content Analysis reader. |
| Animal Protocol |
Mice: Female athymic NMRI nu/nu mice, aged 50 days, with an average body weight of 20-22 g, are utilized for tumor xenograft studies. Animals are randomized to treatment and control groups (8–10 mice / group) when tumors reach a size of 20–40 mm2, depending on the growth of the tumor model. They are then treated p.o. with vehicle (70% polyethylene glycol 400, 5% ethanol, 25% solutol), BAY 1161909, BAY1217389, and/or paclitaxel. A2780cis tumor-bearing female NMRI nude mice are treated with paclitaxel (i.v., once at a dose of 24 mg/kg), BAY 1161909 (p.o., twice daily for two days at a dose of 2.5 mg/kg), and in combination with paclitaxel (i.v., once at a dose of 24 mg/kg) and BAY 1161909 (p.o., twice daily for two days at a dose of 1 mg/kg). This allows for the analysis of polyploidy and multinuclearity induction in vivo.
Pharmacokinetic investigations [2] Pharmacokinetic studies were performed in male Wistar rats and female CD1 or NMRI nu/nu mice. For i.v. studies in rats and mice, Empesertib (BAY1161909) was solubilized in 1% DMSO, 99% plasma; for p.o. studies in rats in 50% polyethylene glycol (PEG) 400, 10% ethanol, 40% water, and for p.o. studies in mice in 75% PEG 400, 5% ethanol, and 25% solutol. BAY1217389 was solubilized in 50% PEG 400, 10% ethanol, and 40% water for intravenous and p.o. dosing in rats and mice. In pharmacokinetic studies, plasma samples were collected after 2, 5, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after intravenous application and after 8, 15, 30, 45 minutes, 1, 2, 4, 6, 8, and 24 hours after p.o. administration and precipitated with ice-cold acetonitrile (1:5). Supernatants were analyzed via LC/MS-MS. Pharmacokinetic parameters were estimated from the plasma concentration data, e.g., using the log-linear trapezoidal rule for AUC estimation. Maximal plasma concentrations (Cmax) and time thereof (Tmax) were taken directly from the concentration time profiles. Animal efficacy studies [2] For tumor xenograft studies, female athymic NMRI nu/nu mice (Taconic), 50 days old, average body weight 20 to 22 g, were used after an acclimatization period of 14 days. Feeding and drinking was ad libitum 24 hours per day. Human tumor cells derived from exponentially growing cell cultures were resuspended for A2780cis, NCI-H1299, and SUM-149 models in 100% Matrigel to a final concentration of 2 × 107, 3 × 107, or 5 × 107 cells/mL, respectively. Subcutaneous implants of 0.1 mL of 2 × 106 A2780cis, 3 × 106 NCI-H1299, or 5 × 106 SUM-149 cells were inoculated into the inguinal region of athymic mice. Tumor fragments of patient tumor explants MAXF 1384 or LU384, obtained from serial passage in nude mice, were cut into fragments of 4 to 5 mm diameter and transplanted subcutaneously into the flank of athymic mice. Tumor area (product of the longest diameter and its perpendicular), measured with a caliper, and body weight were determined two to three times a week. Tumor growth inhibition is presented as treatment/control ratio (T/C) calculated with tumor areas at the end of the study. Animal body weight was used as a measure for treatment-related toxicity. Body weight loss > 20% was dedicated as toxic. When tumors reached a size of approximately 20 to 40 mm², depending on growth of the tumor model, animals were randomized to treatment and control groups (8–10 mice/group) and treated p.o. with vehicle (70% PEG 400, 5 % ethanol, and 25% Solutol), Empesertib (BAY1161909), BAY1217389, and/or paclitaxel, as indicated in Tables and Figure legends. In combination treatment groups, Mps1 inhibitor and paclitaxel were applied at the same day within a time frame of 1 hour. The treatment of each animal was based on individual body weight. In vivo mode of action studies [2] For analysis of polyploidy and multinuclearity induction in vivo, A2780cis tumor–bearing female NMRI nude mice (see above) were treated with paclitaxel (intravenously once with 24 mg/kg), Empesertib (BAY1161909) (p.o. twice daily for 2 days with 2.5 mg/kg), and in combination with paclitaxel (i.v. once 24 mg/kg) and Empesertib (BAY1161909) (p.o. twice daily for 2 days 1 mg/kg). Treatment for all groups started at a tumor size of 60 mm² at day 14 after tumor cell inoculation. Tumor samples were prepared 4 and 8 hours after first Empesertib (BAY1161909) application at treatment day 1, as well as 4, 8, and 24 hours after first application of BAY 1161909 on treatment day 2. At each time point, 3 animals per treatment group were analyzed. Tumors were used for histologic examination after paraffin embedding and hematoxylin and eosin staining. |
| ADME/Pharmacokinetics |
In vivo pharmacokinetic parameters [1] Pharmacokinetic parameters were determined in mouse and rat. Following intravenous administration of BAY 1161909 as bolus of 0.5 mg/kg to male CD1 mouse and 0.5 mg/kg to male Wistar rat, the compound exhibited low blood clearance. The volume of distribution (Vss) was high in both species and terminal half-lives were long. After oral administration of 1 mg/kg to female NMRI mouse and 0.5 mg/kg to male Wistar rat, peak plasma levels were reached after 4 hours. The oral bioavailability was moderate in mouse and rat (Table 1). BAY1217389 was administered intravenously as bolus of 1.0 and 0.5 mg/kg to female CD1 mouse and male Wistar rat, respectively. Blood clearance was found to be low in the tested species. Vss was high and terminal half-lives were long. BAY1217389 was administered orally to female NMRI mouse (1 mg/kg) and male Wistar rat (0.5 mg/kg). Peak plasma concentrations were observed between 1.5 and 7 hours. Oral bioavailability was high in rat and moderate in mouse (Table 1). Our data demonstrate that we have identified two novel Mps1 kinase inhibitors with a favorable pharmacokinetic profile, supporting further development for clinical application. |
| Toxicity/Toxicokinetics |
Purpose: Monopolar spindle 1 (MPS1) kinase inhibitor, BAY 1217389 (BAY) synergizes with paclitaxel. This phase I study assessed the combination of BAY with paclitaxel using a novel randomized continuous reassessment method (rCRM) to improve dose determination.[2] Patients and methods: Patients with solid tumors were randomized to receive oral BAY (twice daily 2-days-on/5-days-off) with weekly paclitaxel (90 mg/m2) or paclitaxel monotherapy in cycle 1. Dose escalation was guided by CRM modeling. Primary objectives were to assess safety, establish the MTD of BAY, and to evaluate the pharmacokinetic profiles for both compounds. Simulations were performed to determine the contribution of the rCRM for dose determination. [2] Results: In total, 75 patients were enrolled. The main dose-limiting toxicities were hematologic toxicities (55.6%). The MTD of BAY was established at 64 mg twice daily with paclitaxel. Inclusion of a control arm enabled the definitive attribution of grade ≥3 neutropenia to higher BAY exposure [AUC0-12 (P< 0.001)]. After determining the MTD, we included 19 patients with breast cancer at this dose for dose expansion. Other common toxicities were nausea (45.3%), fatigue (41.3%), and diarrhea (40.0%). Overall confirmed responses were seen in 31.6% of evaluable patients. Simulations showed that rCRM outperforms traditional designs in determining the true MTD. [2] Conclusions: The combination of BAY with paclitaxel was associated with considerable toxicity without a therapeutic window. However, the use of the rCRM design enabled us to determine the exposure-toxicity relation for BAY. Therefore, we propose that the rCRM could improve dose determination in phase I trials that combine agents with overlapping toxicities. |
| References |
[1]. Novel Mps1 Kinase Inhibitors with Potent Antitumor Activity. Mol Cancer Ther. 2016 Apr;15(4):583-92. [2]. A Phase I Study of an MPS1 Inhibitor (BAY 1217389) in Combination with Paclitaxel Using a Novel Randomized Continual Reassessment Method for Dose Escalation. Clin Cancer Res . 2021 Dec 1;27(23):6366-6375. |
| Additional Infomation | Monopolar spindle 1 (Mps1) has been shown to function as the key kinase that activates the spindle assembly checkpoint (SAC) to secure proper distribution of chromosomes to daughter cells. Here, we report the structure and functional characterization of two novel selective Mps1 inhibitors, BAY 1161909 and BAY 1217389, derived from structurally distinct chemical classes. BAY 1161909 and BAY 1217389 inhibited Mps1 kinase activity with IC50 values below 10 nmol/L while showing an excellent selectivity profile. In cellular mechanistic assays, both Mps1 inhibitors abrogated nocodazole-induced SAC activity and induced premature exit from mitosis ("mitotic breakthrough"), resulting in multinuclearity and tumor cell death. Both compounds efficiently inhibited tumor cell proliferation in vitro (IC50 nmol/L range). In vivo, BAY 1161909 and BAY 1217389 achieved moderate efficacy in monotherapy in tumor xenograft studies. However, in line with its unique mode of action, when combined with paclitaxel, low doses of Mps1 inhibitor reduced paclitaxel-induced mitotic arrest by the weakening of SAC activity. As a result, combination therapy strongly improved efficacy over paclitaxel or Mps1 inhibitor monotreatment at the respective MTDs in a broad range of xenograft models, including those showing acquired or intrinsic paclitaxel resistance. Both Mps1 inhibitors showed good tolerability without adding toxicity to paclitaxel monotherapy. These preclinical findings validate the innovative concept of SAC abrogation for cancer therapy and justify clinical proof-of-concept studies evaluating the Mps1 inhibitors BAY 1161909 and BAY 1217389 in combination with antimitotic cancer drugs to enhance their efficacy and potentially overcome resistance. Mol Cancer Ther; 15(4); 583-92. |
Solubility Data
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (~178.1 mM) Ethanol: ~8 mg/mL (~14.3 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (4.45 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7809 mL | 8.9047 mL | 17.8094 mL | |
| 5 mM | 0.3562 mL | 1.7809 mL | 3.5619 mL | |
| 10 mM | 0.1781 mL | 0.8905 mL | 1.7809 mL |