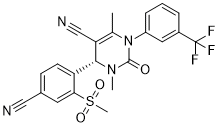
BAY 85-8501 is a novel, highly potent and selective inhibitor of Human Neutrophil Elastase (HNE) with an IC50 of 65 pM. BAY 85-8501 exhibited high in vivo efficacy in various preclinical animal models and is currently being studied in clinical studies for the treatment of pulmonary diseases. Human neutrophil elastase (HNE) is a key protease for matrix degradation. High HNE activity is observed in inflammatory diseases. Accordingly, HNE is a potential target for the treatment of pulmonary diseases such as chronic obstructive pulmonary disease (COPD), acute lung injury (ALI), acute respiratory distress syndrome (ARDS), bronchiectasis (BE), and pulmonary hypertension (PH). HNE inhibitors should reestablish the protease-anti-protease balance. By means of medicinal chemistry a novel dihydropyrimidinone lead-structure class was identified. Further chemical optimization yielded orally active compounds with favorable pharmacokinetics such as the chemical probe BAY-678. While maintaining outstanding target selectivity, picomolar potency was achieved by locking the bioactive conformation of these inhibitors with a strategically positioned methyl sulfone substituent. An induced-fit binding mode allowed tight interactions with the S2 and S1 pockets of HNE. BAY 85-8501 ((4S)-4-[4-cyano-2-(methylsulfonyl)phenyl]-3,6-dimethyl-2-oxo-1-[3-(trifluoromethyl)phenyl]-1,2,3,4-tetrahydropyrimidine-5-carbonitrile) was shown to be efficacious in a rodent animal model related to ALI. BAY 85-8501 is currently being tested in clinical studies for the treatment of pulmonary diseases.
Physicochemical Properties
| Molecular Formula | C22H17F3N4O3S |
| Molecular Weight | 474.455593824387 |
| Exact Mass | 474.097 |
| CAS # | 1161921-82-9 |
| Related CAS # | (R)-BAY-85-8501;2446175-39-7 |
| PubChem CID | 66601502 |
| Appearance | White to off-white solid powder |
| Density | 1.5±0.1 g/cm3 |
| Boiling Point | 626.7±55.0 °C at 760 mmHg |
| Flash Point | 332.8±31.5 °C |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.622 |
| LogP | 1.99 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 33 |
| Complexity | 1030 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | CC1=C([C@H](N(C(=O)N1C2=CC=CC(=C2)C(F)(F)F)C)C3=C(C=C(C=C3)C#N)S(=O)(=O)C)C#N |
| InChi Key | YAJWYFPMASPAMM-HXUWFJFHSA-N |
| InChi Code | InChI=1S/C22H17F3N4O3S/c1-13-18(12-27)20(17-8-7-14(11-26)9-19(17)33(3,31)32)28(2)21(30)29(13)16-6-4-5-15(10-16)22(23,24)25/h4-10,20H,1-3H3/t20-/m1/s1 |
| Chemical Name | (S)-4-(4-cyano-2-(methylsulfonyl)phenyl)-3,6-dimethyl-2-oxo-1-(3-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydropyrimidine-5-carbonitrile |
| Synonyms | BAY 858501; BAY858501; BAY-858501; BAY 85-8501; BAY85-8501; BAY-85-8501. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vivo | In this scenario, the main source of damage and pulmonary bleeding is exogenous HNE noxa. When provided one hour before to HNE noxa, BAY-85-8501 (29) entirely avoided the development of lung damage and subsequent inflammation, based on picomolar potency against HNE and single-digit potency against MNE. There has been a substantial decrease in hemoglobin concentration in the 0.01 mg/kg dosage group. There was a noticeable impact on neutrophil counts at 0.1 mg/kg. In this situation, potency against HNE (Ki=0.08 nM) was the main factor influencing efficacy. In this context, BAY-85-8501, a highly HNE-selective inhibitor, does not prevent primary lung injury since it has no effect on PPE. Though less potently, BAY-85-8501 can block MNE, an endogenous driver of inflammation and secondary damage. As a result, BAY-85-8501 now has a minimal impact on inflammation and secondary damage and is only noticeable at doses that are thirty times higher. Potency against MNE (Ki=6 nM) is the primary factor influencing efficacy in the second setting [1]. |
| References |
[1]. Freezing the Bioactive Conformation to Boost Potency: The Identification of BAY 85-8501, a Selective and Potent Inhibitor of Human Neutrophil Elastase for Pulmonary Diseases. ChemMedChem. 2015 Jul;10(7):1163-73. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~200 mg/mL (~421.53 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (10.54 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (10.54 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 2.5 mg/mL (5.27 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1077 mL | 10.5383 mL | 21.0766 mL | |
| 5 mM | 0.4215 mL | 2.1077 mL | 4.2153 mL | |
| 10 mM | 0.2108 mL | 1.0538 mL | 2.1077 mL |