BAN ORL 24 is a novel, potent and selective NOP (nociceptin/orphanin FQ (N/OFQ) peptide receptor) receptor antagonist with IC50 values of 0.27, 2500, 6700 and > 10000 nM for NOP, κ-, μ- and δ-receptors respectively. Compound 24 (BAN ORL 24) did not change the way classical opioid receptor agonists worked even at concentrations of up to 1 microM. Lastly, in vivo, Compound 24 at 10 mg/kg countered the pronociceptive and antinociceptive effects of 1 nmol N/OFQ administered spinally and supraspinally, respectively, in the mouse tail withdrawal assay. Compound 24 had no effect on the intrathecal injection of 3 nmol endomorphin-1's antinociceptive action under the same experimental conditions. Compound 24 is a pure, competitive, and extremely powerful non-peptide NOP receptor selective antagonist, as the current study has shown.
Physicochemical Properties
| Molecular Formula | C27H37CL2N3O2 |
| Molecular Weight | 506.507585287094 |
| Exact Mass | 505.226 |
| Elemental Analysis | C, 64.03; H, 7.36; Cl, 14.00; N, 8.30; O, 6.32 |
| CAS # | 1401463-54-4 |
| Related CAS # | BAN ORL 24 free base; 475150-69-7 |
| PubChem CID | 56840444 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 34 |
| Complexity | 612 |
| Defined Atom Stereocenter Count | 1 |
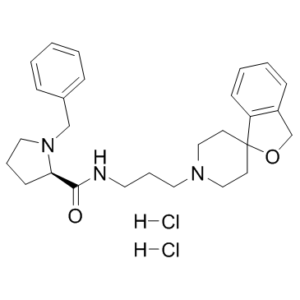
| SMILES | O=C([C@@H]1N(CC2=CC=CC=C2)CCC1)NCCCN3CCC4(CC3)OCC5=C4C=CC=C5.[H]Cl.[H]Cl |
| InChi Key | NEEVITHVDIQNJY-KHZPMNTOSA-N |
| InChi Code | InChI=1S/C27H35N3O2.2ClH/c31-26(25-12-6-17-30(25)20-22-8-2-1-3-9-22)28-15-7-16-29-18-13-27(14-19-29)24-11-5-4-10-23(24)21-32-27;;/h1-5,8-11,25H,6-7,12-21H2,(H,28,31);2*1H/t25-;;/m1../s1 |
| Chemical Name | (2R)-1-benzyl-N-(3-spiro[1H-2-benzofuran-3,4'-piperidine]-1'-ylpropyl)pyrrolidine-2-carboxamide;dihydrochloride |
| Synonyms | BAN-ORL-24 HCl; BAN-ORL-24 dihydrochloride; BAN-ORL24; BAN-ORL 24; BANORL-24; BANORL 24; BANORL24; BAN ORL 24 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | BAN ORL 24 has NOR and MOR (opioid receptor subtype) antagonists with IC50 values of 50 μM and 0.224 μM respectively [2]. |
| ln Vivo | BAN ORL 24 (10 mg/kg; intravenously) attenuates the duration of BPRIM97 thermal analgesia [3]. Animal model: C57BL/6 mice [3] Dose: 10 mg/kg Administration dose: 10 mg/kg; iv results: Inhibited BPRIM97-induced analgesia 90 minutes after injection. BPR1M97-induced analgesia was not attenuated in the tail-clip test after 30 minutes. |
| References |
[1]. (2009) Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist compound 24. Eur.J.Pharmacol. 614 50. [2]. Label-free cell phenotypic study of opioid receptors and discovery of novel mu opioid ligands from natural products. J Ethnopharmacol. [3]. BPR1M97, a dual mu opioid receptor/nociceptin-orphanin FQ peptide receptor agonist, produces potent antinociceptive effects with safer properties than morphine. Neuropharmacology 166, 107678 (2020). |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~200 mg/mL (~394.86 mM) H2O : ~100 mg/mL (~197.43 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 5 mg/mL (9.87 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 5 mg/mL (9.87 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 5 mg/mL (9.87 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 50.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 100 mg/mL (197.43 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9743 mL | 9.8715 mL | 19.7429 mL | |
| 5 mM | 0.3949 mL | 1.9743 mL | 3.9486 mL | |
| 10 mM | 0.1974 mL | 0.9871 mL | 1.9743 mL |