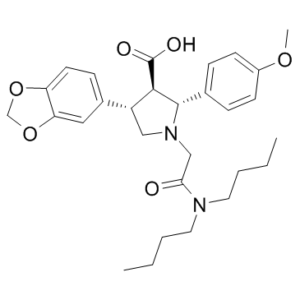
Atrasentan (ABT-627; NSC720763; A-147627; trade name: Xinlay) is a novel, selective and potent endothelin receptor/ET antagonist with anticancer activity. It suppresses ETA with an IC50 of 0.0551 nM. It's an experimental medication being researched to treat different cancers, including non-small cell lung cancer. Additionally, it's being looked into as a treatment for diabetic kidney disease. In patients who were not responding to hormone therapy, trasentan was not successful in a phase 3 trial for prostate cancer. Atrasentan is a medication that blocks the function of endothelin-1 and may be used to treat hormone-resistant prostate cancer that has metastasized.
Physicochemical Properties
| Molecular Formula | C29H38N2O6 |
| Molecular Weight | 510.62182 |
| Exact Mass | 510.273 |
| Elemental Analysis | C, 68.21; H, 7.50; N, 5.49; O, 18.80 |
| CAS # | 173937-91-2 |
| Related CAS # | Atrasentan hydrochloride; 195733-43-8; 178738-96-0 (sodium); 195704-72-4 |
| PubChem CID | 159594 |
| Appearance | White to light yellow solid powder |
| Density | 1.188g/cm3 |
| Boiling Point | 659.4ºC at 760mmHg |
| Flash Point | 352.6ºC |
| Vapour Pressure | 2.76E-18mmHg at 25°C |
| LogP | 4.631 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 12 |
| Heavy Atom Count | 37 |
| Complexity | 734 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | O=C([C@H]1[C@H](C2=CC=C(OC)C=C2)N(CC(N(CCCC)CCCC)=O)C[C@@H]1C3=CC=C(OCO4)C4=C3)O |
| InChi Key | MOTJMGVDPWRKOC-QPVYNBJUSA-N |
| InChi Code | InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 |
| Chemical Name | (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid |
| Synonyms | ABT-627; (+)-A 127722; ABT627; ABT 627; ABT-627; NSC720763; A147627; Abbott 147627; trade name: Xinlay; A-147627 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | ETA ( IC50 = 0.055 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | Atrasentan is used to treat and incubate the cells. After two PBS washes, they are lysed in an ice-cold lysis buffer [1 mM EGTA, 1 mM EDTA, 1.5 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium PPi, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/mL leupeptin, and 1 mM PMSF]. After centrifuging the extracts to get rid of any remaining cell debris, the bicinchoninic acid (BCA) protein assay reagent is used to measure the amount of protein in the supernatants. Proteins (150 μg) cross-linked to agarose hydrazide beads and incubated with mild rocking at 4°C for the entire night. The lysis buffer and kinase assay buffer (25 mM Tris (pH 7.5), 10 mM MgCl2, 5 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 2 mM DTT) are used to wash the immunoprecipitated products twice after Akt is selectively immunoprecipitated from the cell lysates. The products are then resuspended in 40 μL of kinase assay buffer that contains 200 μM ATP and 1 μg GSK-3α/β fusion protein. The addition of Lamelli SDS sample buffer stops the kinase assay reaction after it has been running for 30 minutes at 30°C. 10% SDS-PAGE is used to resolve the reaction products, and then an antiphosphorylated GSK-3α/β antibody is used for Western blotting. After separating 40 μg of protein from the lysate samples using 10% SDS-PAGE, anti-Akt antibody is used for Western blotting in order to determine the total amount of Akt. | |
| Cell Assay |
|
|
| Animal Protocol |
|
|
| References |
[1]. Superiority of YM598 over atrasentan as a selective endothelin ETA receptor antagonist. Eur J Pharmacol. 2004 Sep 13;498(1-3):171-7. [2]. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 combination in prostate cancer. Cancer Res. 2007 Apr 15;67(8):3818-26. [3]. Interaction potential of the endothelin-A receptor antagonist atrasentan with drug transporters and drug-metabolising enzymes assessed in vitro. Cancer Chemother Pharmacol. 2011 Oct;68(4):1093-8. |
|
| Additional Infomation |
Atrasentan is a member of pyrrolidines. Atrasentan is a substance that is being studied in the treatment of cancer. It belongs to the family of drugs called endothelin-1 protein receptor antagonists. It is a novel, selective endothelin A receptor antagonist (SERA). A pyrrolidine and benzodioxole derivative that acts a RECEPTOR, ENDOTHELIN A antagonist. It has therapeutic potential as an antineoplastic agent and for the treatment of DIABETIC NEPHROPATHIES. Drug Indication Investigated for use/treatment in prostate cancer and cancer/tumors (unspecified). |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9584 mL | 9.7920 mL | 19.5840 mL | |
| 5 mM | 0.3917 mL | 1.9584 mL | 3.9168 mL | |
| 10 mM | 0.1958 mL | 0.9792 mL | 1.9584 mL |