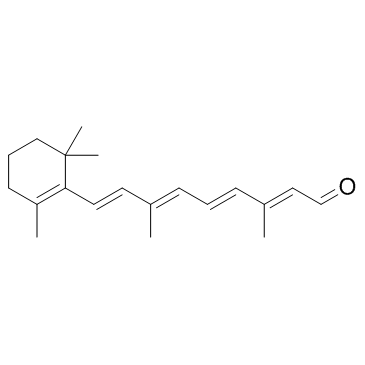
All-trans-retinal (Retinaldehyde; NSC-626581; Retinal A; NSC-122757; Vitamin A aldehyde) is one of the major vitamin A metabolites in the retina. It is an oxidized form of retinol, which is a corotenoid component of the visual pigments. In physiological conditions, all-trans-RAL is regenerated to the visual chromophore, 11-cis-retinal.
Physicochemical Properties
| Molecular Formula | C₂₀H₂₈O |
| Molecular Weight | 284.44 |
| Exact Mass | 284.214 |
| Elemental Analysis | C, 84.45; H, 9.92; O, 5.62 |
| CAS # | 116-31-4 |
| PubChem CID | 638015 |
| Appearance | Light yellow to yellow solid |
| Density | 0.9±0.1 g/cm3 |
| Boiling Point | 421.4±14.0 °C at 760 mmHg |
| Melting Point | 61-63°C |
| Flash Point | 205.4±12.4 °C |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.541 |
| Source | Gut microbes |
| LogP | 6.54 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 21 |
| Complexity | 522 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O=C([H])/C(/[H])=C(\C([H])([H])[H])/C(/[H])=C(\[H])/C(/[H])=C(\C([H])([H])[H])/C(/[H])=C(\[H])/C1=C(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])C1(C([H])([H])[H])C([H])([H])[H] |
| InChi Key | NCYCYZXNIZJOKI-OVSJKPMPSA-N |
| InChi Code | InChI=1S/C20H28O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,15H,7,10,14H2,1-5H3/b9-6+,12-11+,16-8+,17-13+ |
| Chemical Name | (2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenal |
| Synonyms | Retinaldehyde; NSC-626581; Retinal; ARetinal; Vitamin A aldehyde; Retinaldehyde; NSC 122757; NSC626581; NSC122757; NSC-626581; NSC-122757; NSC 626581; all-trans-Retinal; retinene; axerophthal; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture.(3). This product is not stable in solution, please use freshly prepared working solution for optimal results. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Endogenous Metabolite |
| ln Vitro | Incubation with Bax inhibiting peptide and deletion of the Bax gene partially protected retinal cells from atRAL toxicity in cultured neural retina. Necrosis was demonstrated not to be the main pathway in atRAL mediated cell death. Bcl-2-interacting mediator and Bcl-2 expression levels were not altered by atRAL in vitro. atRAL-induced oxidative stress results in DNA damage leading to the activation of Bax by phosphorylated p53 [1]. |
| ln Vivo | Other Bax-related cellular events were also evaluated by pharmacological and biochemical methods. Production of 8-OHdG, a DNA damage indicator, and the phosphorylation of p53 at Ser46 were detected prior to Bax activation in ARPE-19 cells incubated with atRAL. Light exposure to Abca4(-/-)Rdh8(-/-) mice also caused the above mentioned events in conditions of short term intense light exposure and regular room lighting conditions [1]. |
| Enzyme Assay | Lactate Dehydrogenase (LDH) Colorimetric Assay LDH measurements were conducted using LDH-Cytotoxicity Colorimetric Assay Kit II (BioVision, Milpitas, CA) on cultured ARPE-19 cells and 661W cells in 96 well plates (104 cells/ well) following manufacturer’s instructions. Optical density values from the 96-well plate at 450 nm wavelength were measured with a microplate reader [1]. |
| Cell Assay |
WST-1 assay [1] WST-1 assay was conducted with Cell Proliferation Reagent WST-1 on ARPE-19 cells cultured in 96-well plates (1 × 104 cells/ well) following manufacturer’s instructions. Optical density values from the 96-well plate at 450 nm wavelength were measured with a microplate reader. In vitro detection of Bax activation[1] ARPE-19 cells in 24 well cell plates (1 × 105 cells/well) were treated with atRAL at a final concentration of 10 – 30 μM in the cell culture medium for the indicated time with or without five mar Bax inhibiting peptide, BIP. Cells were washed in cold PBS and kept on ice after incubation. Cells were then fixed for 30 min with 4% paraformaldehyde prior to permeabilization with 0.5% Triton X at room temperature. Bax activation was detected via immunocytochemistry (ICC) with anti-Bax (6A7), a mouse monoclonal antibody at a dilution of 1:100. Cy-3 conjugated anti-mouse IgG antibody was the secondary antibody and was used at a dilution of 1:200. The signal intensity of activated Bax was monitored with an inverted fluorescence microscope. In vitro detection of phosphorylated p53 at Ser46[1] ARPE-19 cells were cultured in 24 well plates (1 × 105 cells/well) and atRAL was added at a final concentration of 30 μM then incubated for 30 min. Phosphorylation of p53 at Ser46 was detected by ICC with anti-phosphorylated p53 (Ser46) specific antibody (rabbit polyclonal, at 1:50 dilution). Cy-3 conjugated anti-rabbit IgG antibody was used as the secondary antibody. Signal intensity measurements of phosphorylated p53 were also performed. In vitro detection of 8-OHdG[1] ARPE-19 cells in 24 well plates (1 × 105 cells/well) were incubated with atRAL at the indicated concentration (10 – 30 μM) for 30 min. The marker of DNA injury by oxidative stress, 8-hydroxyhydroguanidine (8-OHdG) was monitored using ICC. Cells were incubated for 1 h with anti-8-OHdG antibody at a concentration of 10 μg/ml. Cy-3 conjugated anti-mouse IgG antibody was used as the secondary antibody. Mitochondria-selective Staining[1] ARPE-19 cells in 96 well plates (1 × 104 cells/well) were incubated with Mitochondrion-Selective Probes, MitoTracker Orange CMTMRos at 100 nM for 30 min. |
| Animal Protocol |
Animals[1] Abca4 −/−Rdh8−/− mice and Bax−/− mice were generated and genotyped as previously described (Maeda et al., 2008). C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor Maine). All mice in this study were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were regularly maintained in a 12 h light (~10 lux) /12 h dark cycle environment. All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees and conformed to both the recommendations of the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology. Induction of light damage[1] Light damage was induced in mice by fluorescent light exposure at 10,000 lux (150 W spiral lamps, Commercial Electric) for 30 min in a white bucket as previously described (Maeda et al., 2012). The mice were dark-adapted overnight and pupils were dilated with 1% tropicamide eye solution in prior to light exposure. Measurement of spectral-domain optical coherence tomography (SD-OCT)[1] SD-OCT was employed for in vivo retinal imaging of mice as previously described |
| ADME/Pharmacokinetics |
Metabolism / Metabolites Retinal has known human metabolites that include Tretinoin. Retinal is a known human metabolite of retinol. |
| References |
[1]. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp Eye Res. 2014 Jun;123:27-36. |
| Additional Infomation |
All-trans-retinal is a retinal in which all four exocyclic double bonds have E- (trans-) geometry. It has a role as a gap junctional intercellular communication inhibitor, a human metabolite and a mouse metabolite. It is a retinal and a vitamin A. Retinal is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). Retinal has been reported in Suberites domuncula, Homo sapiens, and other organisms with data available. A diterpene derived from the carotenoid VITAMIN A which functions as the active component of the visual cycle. It is the prosthetic group of RHODOPSIN (i.e., covalently bonded to ROD OPSIN as 11-cis-retinal). When stimulated by visible light, rhodopsin transforms this cis-isomer of retinal to the trans-isomer (11-trans-retinal). This transformation straightens-out the bend of the retinal molecule and causes a change in the shape of rhodopsin triggering the visual process. A series of energy-requiring enzyme-catalyzed reactions convert the 11-trans-retinal back to the cis-isomer. See also: 13-cis-Retinal (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~351.57 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (7.31 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (7.31 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (7.31 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.5157 mL | 17.5784 mL | 35.1568 mL | |
| 5 mM | 0.7031 mL | 3.5157 mL | 7.0314 mL | |
| 10 mM | 0.3516 mL | 1.7578 mL | 3.5157 mL |