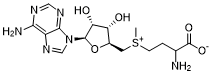
Ademetionine (AdoMet; SAMe; MSI-195; S-adenosylmethionine) is a common co-substrate involving in the transfer of methyl group to biological molecules such as nucleic acids, proteins and lipids. It can be potentially used for treatment of primary biliary cirrhosis and major depressive disorder.
Physicochemical Properties
| Molecular Formula | C15H22N6O5S |
| Molecular Weight | 398.438 |
| Exact Mass | 398.137 |
| Elemental Analysis | C, 45.22; H, 5.57; N, 21.09; O, 20.08; S, 8.05 |
| CAS # | 29908-03-0 |
| Related CAS # | S-Adenosyl-L-methionine tosylate;52248-03-0;S-Adenosyl-L-methionine-d3;68684-40-2;S-Adenosyl-L-methionine disulfate tosylate;97540-22-2;S-Adenosyl-L-methionine iodide;3493-13-8;S-Adenosyl-L-methionine (1,4-butanedisulfonate);200393-05-1;S-Adenosyl-L-methionine-13C;74084-24-5 |
| PubChem CID | 34755 |
| Appearance | Off-white to light yellow solid powder |
| Melting Point | 247-249ºC |
| LogP | -2.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 27 |
| Complexity | 527 |
| Defined Atom Stereocenter Count | 5 |
| SMILES | C[S+](CC[C@@H](C(=O)[O-])N)C[C@@H]1[C@H]([C@H]([C@@H](O1)N2C=NC3=C(N=CN=C32)N)O)O |
| InChi Key | MEFKEPWMEQBLKI-AIRLBKTGSA-N |
| InChi Code | InChI=1S/C15H22N6O5S/c1-27(3-2-7(16)15(24)25)4-8-10(22)11(23)14(26-8)21-6-20-9-12(17)18-5-19-13(9)21/h5-8,10-11,14,22-23H,2-4,16H2,1H3,(H2-,17,18,19,24,25)/t7-,8+,10+,11+,14+,27?/m0/s1 |
| Chemical Name | (2S)-2-amino-4-((((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(methyl)sulfonio)butanoate |
| Synonyms | S-adenosylmethionine; MSI195; AdoMet; S-adenosylmethionine; Active methionine; S-adenosyl methionine; Adenosine, 5'-((3-amino-3-carboxypropyl)methylsulfonio)-5'-deoxy-, inner salt, (3S)-; DTXSID6032019; S-adenosyl-methionine; S Adenosylmethionine; ...; 29908-03-0; MSI-195; SAMeMSI 195; Ademetionine; Heptral Gumbaral. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | S-adenosyl-L-methionine (SAMe) acts through multiple molecular targets, including: - DNA methyltransferases (DNMTs) (e.g., DNMT1, DNMT3A) in cancer cells, where it modulates DNA methylation and enhances 5-fluorouracil (5-FU) efficacy . - Histone deacetylases (HDACs) and methyltransferases in epigenetic regulation of gene expression, particularly in liver disease and cancer . |
| ln Vitro |
In Cal-33 and JHU-SCC-011 cells, S-adenosyl-L-methionine (300 µM, 24 or 48 hours) activates cells and encourages cell cycle marketing [4]. S-adenosyl-L-methionine (300 S-adenosyl-L-methionine (5–40 μg/mL, 48 h) inhibits Cal–33 and JHU-SCC-011 cell migration while protecting 5‑FU migration by controlling DNMT expression (μM, 24 h) [4]. Analysis of apoptosis [4]
1. Antiproliferative Activity: - In human A549 lung cancer cells, SAMe (1–10 mM) combined with 5-FU (10 μM) significantly reduced cell viability compared to 5-FU alone, as measured by MTT assay. This effect was associated with upregulation of DNMTs expression, enhancing DNA methylation and apoptosis . - In head and neck squamous cancer cells (CAL-33 and JHU-SCC-011), SAMe (0.5–2 mM) dose-dependently inhibited cell migration and invasion, as assessed by Transwell and Matrigel assays. Western blot analysis revealed downregulation of AKT and β-catenin signaling pathways . 2. Enzyme Modulation: - SAMe (1–10 μM) increased DNMTs activity in A549 cells, protecting the anticancer effect of 5-FU by stabilizing DNA methylation patterns . - In synovial cells, SAMe (10–100 μM) restored TNF-α-induced reduction in proteoglycan synthesis, suggesting chondroprotective effects in osteoarthritis . |
| ln Vivo |
S-adenosyl-L-methionine (30 mg/kg, facial, for 3 days) prevents ASD-like behaviors caused by valproic acid exposure in early postnatal rats [6]. S-adenosyl-L-methionine (50 and 100)
1. Tumor Growth Inhibition: - In athymic nude mice bearing A549 lung tumors, SAMe (100 mg/kg/day, oral) combined with 5-FU (20 mg/kg, intraperitoneal) significantly reduced tumor volume compared to 5-FU alone. Histological analysis showed increased necrosis and reduced Ki-67 staining . 2. Neuroprotective Effects: - In a valproic acid (VPA)-induced autism-like mouse model, SAMe (30 mg/kg/day, oral) prevented social deficits and repetitive behaviors, with normalization of oxidative stress markers (e.g., SOD, catalase) in the prefrontal cortex . 3. Antiepileptic Effects: - In pentylenetetrazole (PTZ)-kindled rats, SAMe (100 mg/kg, intraperitoneal) prolonged seizure latency and reduced seizure severity. Memory impairment was reversed, as demonstrated by improved performance in the elevated plus maze test . |
| Enzyme Assay | 1. DNMTs Activity Assay: - A549 cell lysates were incubated with SAMe (1–10 μM) and [³H]-methyl-SAMe. DNA methylation was quantified by liquid scintillation counting. SAMe increased DNMTs activity in a dose-dependent manner, with maximal effect at 5 μM . 2. Antioxidant Enzyme Assay: - Brain homogenates from VPA-exposed mice treated with SAMe were analyzed for SOD and catalase activity. SAMe restored enzyme activity to control levels, indicating antioxidant effects . |
| Cell Assay |
Apoptosis analysis [4] Cell Types: Cal-33 and JHU-SCC-011 cells Tested Concentrations: 300 μM Incubation Duration: 24 hrs (hours) (Cal-33) or 48 hrs (hours) ( HU-SCC-011) results. Anti-cancer effect [5]. Experimental Results: demonstrated approximately 10% and 3% of apoptotic cells respectively. Cell cycle analysis [4] Cell Types: Cal-33 and JHU-SCC-011 Cell Tested Concentrations: 300 µM Incubation Duration: 24 hrs (hours) (Cal-33) or 48 hrs (hours) (HU-SCC-011) Experimental Results: Cyclin expression diminished B1, E1, and D1 in Cal-33 and JHU-SCC-011 cells. |
| Animal Protocol |
Animal/Disease Models: Valproic acid-treated young rats [6] Doses: 30 mg/kg Route of Administration: Oral for 3 days Experimental Results: Remission of most autism spectrum disorders (ASD)-like neurobehavioral symptoms. Normalizes redox potential in the prefrontal cortex. 1. Tumor Xenograft Model: - A549 cells (5 × 10⁶) were implanted subcutaneously in nude mice. SAMe (100 mg/kg) was administered orally daily, and 5-FU (20 mg/kg) intraperitoneally twice weekly. Tumor volume was measured twice weekly using calipers . 2. Autism-like Mouse Model: - Neonatal ICR mice received VPA (300 mg/kg, intraperitoneal) on postnatal day 4. SAMe (30 mg/kg) was administered orally from day 5 to 7. Behavioral tests (e.g., social interaction, marble burying) were conducted at day 50 . 3. PTZ-Kindling Model: - Male Wistar rats received PTZ (35 mg/kg, intraperitoneal) every other day for 14 days to induce kindling. SAMe (100 mg/kg) was administered intraperitoneally 30 minutes before PTZ. Seizure severity was scored using Racine’s scale, and memory was assessed via elevated plus maze . |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion S-Adenosylmethionine is absorbed from the small intestine following oral intake. As absorption is affected by food, it is best to take on an empty stomach. Bioavailability is low following oral intake. Metabolism / Metabolites Significant first-pass metabolism in the liver. Approximately 50% of S-Adenosylmethionine (SAMe) is metabolized in the liver. SAMe is metabolized to S-adenosylhomocysteine, which is then metabolized to homocysteine. Homocysteine can either be metabolized to cystathionine and then cysteine or to methionine. The cofactor in the metabolism of homocysteine to cysteine is vitamin B6. Cofactors for the metabolism of homocysteine to methionine are folic acid, vitamin B12 and betaine. 1. Absorption: - Oral bioavailability of SAMe is low (~5–10%) due to extensive first-pass metabolism in the liver . - In rats, peak plasma concentration (Cmax) of SAMe (100 mg/kg, oral) was 2.1 ± 0.3 μM at 1 hour post-dose . 2. Distribution: - SAMe readily crosses the blood-brain barrier, with brain/plasma concentration ratio of 0.8–1.2 in mice . 3. Metabolism: - SAMe is metabolized to S-adenosylhomocysteine (SAH) by methyltransferases and further to homocysteine . 4. Excretion: - Approximately 70–80% of the dose is excreted in urine as metabolites within 24 hours . |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation SAM-e (S-adenosylmethionine) is a naturally occurring methyl radical donor involved in enzymatic transmethylation reactions in humans and animals. SAM-e has no specific lactation-related uses, but it has been used therapeutically for treating postpartum depression, cholestatic jaundice, osteoarthritis and numerous other conditions. SAM-e has poor oral bioavailability. SAM-e is generally well tolerated in adults. The most frequent adverse effects reported are gastrointestinal, such as nausea. Skin rashes have also been reported. No information is available on the clinical use of SAM-e during breastfeeding. However, use of SAM-e by a nursing mother would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to prove the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does not certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information about dietary supplements is available elsewhere on the LactMed Web site. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Toxicity Summary S-Adenosylmethionine (SAMe) is a natural substance present in the cells of the body. It is a direct metabolite of the essential amino acid L-methionine. SAMe plays a crucial biochemical role in the body by donating a one-carbon methyl group in a process called transmethylation. SAMe, formed from the reaction of L-methionine and adenosine triphosphate catalyzed by the enzyme S-adenosylmethionine synthetase, is the methyl-group donor in the biosynthesis of both DNA and RNA nucleic acids, phospholipids, proteins, epinephrine, melatonin, creatine and other molecules. 1. Acute Toxicity: - LD₅₀ of SAMe in mice is >5 g/kg (oral), with no significant adverse effects at doses up to 1000 mg/kg . - In rats, single-dose SAMe (1000 mg/kg, oral) caused transient gastrointestinal upset but no organ damage . 2. Chronic Toxicity: - Long-term administration (6 months) of SAMe (200 mg/kg/day, oral) in dogs showed no hematological or biochemical abnormalities . 3. Drug Interactions: - SAMe may enhance the hepatotoxicity of methotrexate in rats, likely via competition for hepatic transport proteins . |
| References |
[1]. G M Bressa. S-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand Suppl. 1994;154:7-14. [2]. S-adenosyl methionine (SAMe) versus celecoxib for the treatment of osteoarthritis symptoms: a double-blind cross-over trial. [ISRCTN36233495]. BMC Musculoskelet Disord. 2004 Feb 26;5:6. [3]. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012 Oct;92(4):1515-42. [4]. Effects of S‑adenosyl‑L‑methionine on the invasion and migration of head and neck squamous cancer cells and analysis of the underlying mechanisms. Int J Oncol. 2020 May;56(5):1212-1224. [5]. S-adenosyl methionine specifically protects the anticancer effect of 5-FU via DNMTs expression in human A549 lung cancer cells. Mol Clin Oncol. 2013 Mar;1(2):373-378. [6]. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicol Teratol. 2019 Jan-Feb;71:64-74. [7]. Evaluation of antiepileptic effect of S-adenosyl methionine and its role in memory impairment in pentylenetetrazole-induced kindling model in rats. Epilepsy Behav. 2016 Aug;61:153-157. |
| Additional Infomation |
S-adenosyl-L-methioninate is a sulfonium betaine that is a conjugate base of S-adenosyl-L-methionine obtained by the deprotonation of the carboxy group. It has a role as a human metabolite. It is functionally related to a L-methioninate. It is a conjugate base of a S-adenosyl-L-methionine. Physiologic methyl radical donor involved in enzymatic transmethylation reactions and present in all living organisms. It possesses anti-inflammatory activity and has been used in treatment of chronic liver disease. (From Merck, 11th ed) S-Adenosylmethionine is a metabolite found in or produced by Escherichia coli (strain K12, MG1655). S-adenosylmethionine has been reported in Homo sapiens and Pisum sativum with data available. S-Adenosylmethionine is a nutritional supplement that is synthesized from adenosine triphosphate (ATP) and the amino acid methionine by the endogenous essential enzyme methionine adenosyltransferase (MAT), with potential antineoplastic activity. Upon administration, S-adenosylmethionine acts as a methyl donor for various transmethylation reactions. In cancer cells, this agent induces the methylation of tumor promoting genes, reverses DNA hypomethylation, and leads to the suppression of oncogene transcription. This induces apoptosis in and inhibits proliferation of susceptible tumor cells. Physiologic methyl radical donor involved in enzymatic transmethylation reactions and present in all living organisms. It possesses anti-inflammatory activity and has been used in treatment of chronic liver disease. (From Merck, 11th ed) See also: S-Adenosyl-L-methionine Disulfate Tosylate (active moiety of); S-Adenosylmethionine chloride (is active moiety of). Drug Indication S-Adenosylmethionine (SAMe) is used as a drug in Europe for the treatment of depression, liver disorders, fibromyalgia, and osteoarthritis. It has also been introduced into the United States market as a dietary supplement for the support of bone and joint health, as well as mood and emotional well being. Mechanism of Action S-Adenosylmethionine (SAMe) is a natural substance present in the cells of the body. It is a direct metabolite of the essential amino acid L-methionine. SAMe plays a crucial biochemical role in the body by donating a one-carbon methyl group in a process called transmethylation. SAMe, formed from the reaction of L-methionine and adenosine triphosphate catalyzed by the enzyme S-adenosylmethionine synthetase, is the methyl-group donor in the biosynthesis of both DNA and RNA nucleic acids, phospholipids, proteins, epinephrine, melatonin, creatine and other molecules. Pharmacodynamics S-adenosylmethionine is an intermediate metabolite of methionine. Its involvement in methylation assists in cellular growth and repair, maintains the phospho-bilipid layer in cell membranes. It also helps in the maintenance of the action of several hormones and neurotransmitters that affect mood. Highest concentration are found in the brain and the liver. - Mechanism of Action: SAMe acts as a methyl donor for DNA, histones, and neurotransmitters, regulating epigenetic processes and redox balance. In cancer, it sensitizes cells to chemotherapies by modulating DNMTs . - Clinical Uses: Approved in Europe for depression, osteoarthritis, and liver disease; available as a dietary supplement in the U.S. . - FDA Status: Not approved as a drug in the U.S. but regulated as a dietary supplement. No specific FDA warnings for SAMe alone, but interactions with antidepressants (e.g., SSRIs) may increase serotonin levels . - Preclinical Efficacy: SAMe shows synergistic effects with 5-FU in lung cancer models and prevents autism-like behaviors in mice . |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~100 mg/mL (~250.98 mM) H2O : ≥ 43 mg/mL (~107.92 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5098 mL | 12.5489 mL | 25.0979 mL | |
| 5 mM | 0.5020 mL | 2.5098 mL | 5.0196 mL | |
| 10 mM | 0.2510 mL | 1.2549 mL | 2.5098 mL |