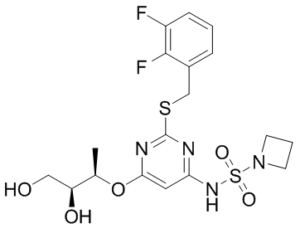
AZD-5069 (AZD5069) is a novel and potent CXCR2 chemokine receptor antagonist with potential anticancer and antiinflammatory activities. The proliferation and progression of tumor cells are facilitated by the upregulation of the CXC chemokine receptor CXCR2 in a range of distinct tumor cell types. Reduced tumorigenesis and metastasis were the results of CXCR2 inhibition. At a pIC50 of 9.1, AZD-5069 prevents radiolabeled CXCL8 from binding to human CXCR2. In phase Ib/II studies, AZD5069, an antagonist of CXCR2, is presently being studied in combination with tremelimumab for patients with metastatic squamous cell carcinoma of the head and neck and advanced solid tumors. AZD-5069 has the potential to treat individuals suffering from COPD and other inflammatory diseases.
Physicochemical Properties
| Molecular Formula | C18H22F2N4O5S2 | |
| Molecular Weight | 476.51 | |
| Exact Mass | 476.099 | |
| Elemental Analysis | C, 45.37; H, 4.65; F, 7.97; N, 11.76; O, 16.79; S, 13.46 | |
| CAS # | 878385-84-3 | |
| Related CAS # |
|
|
| PubChem CID | 56645576 | |
| Appearance | White to off-white solid powder | |
| Density | 1.6±0.1 g/cm3 | |
| Boiling Point | 680.5±65.0 °C at 760 mmHg | |
| Flash Point | 365.4±34.3 °C | |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C | |
| Index of Refraction | 1.651 | |
| LogP | 2.43 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 12 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 31 | |
| Complexity | 670 | |
| Defined Atom Stereocenter Count | 2 | |
| SMILES | N(C1C=C(O[C@H](C)[C@@H](O)CO)N=C(SCC2C=CC=C(F)C=2F)N=1)S(N1CCC1)(=O)=O |
|
| InChi Key | QZECRCLSIGFCIO-RISCZKNCSA-N | |
| InChi Code | InChI=1S/C18H22F2N4O5S2/c1-11(14(26)9-25)29-16-8-15(23-31(27,28)24-6-3-7-24)21-18(22-16)30-10-12-4-2-5-13(19)17(12)20/h2,4-5,8,11,14,25-26H,3,6-7,9-10H2,1H3,(H,21,22,23)/t11-,14+/m1/s1 | |
| Chemical Name | N-[2-[(2,3-difluorophenyl)methylsulfanyl]-6-[(2R,3S)-3,4-dihydroxybutan-2-yl]oxypyrimidin-4-yl]azetidine-1-sulfonamide | |
| Synonyms | AZD 5069; AZD-5069; AZD5069 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | 125I-IL-8-CXCR2 | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | AZD-5069 functions as a CXCR2 antagonist by preventing radiolabelled [125I]-IL-8 from binding to human CXCR2 receptors. It also prevents GROα-induced Ca2+ flux in human neutrophils that have been loaded with fluo-3 dye. | ||
| Cell Assay | In a humidified incubator at 37°C and 5% CO2, human embryonic kidney 293 (HEK293) cells expressing recombinant human CXCR2 or CXCR1 were grown to about 80% confluence in Dulbecco's modified Eagle’s medium–Glutamax medium (Life Technologies Ltd, Paisley, UK) containing 10% (v/v) fetal calf serum and 0.5 mg/ml geneticin. Accutase (Sigma-Aldrich Company Ltd., Dorset, UK) was used to extract cells from the flask after it had been at 37°C for three to five minutes. | ||
| Animal Protocol |
|
||
| References |
[1]. AZD8797 is an allosteric non-competitive modulator of the human CX3CR1 receptor. Biochem J. 2016 Mar 1;473(5):641-9. [2]. Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc Natl Acad Sci U S A. 2014 Apr 8;111(14):5409-14. [3]. Substituted 7-amino-5-thio-thiazolo[4,5-d]pyrimidines as potent and selective antagonists of the fractalkine receptor (CX3CR1). J Med Chem. 2013 Apr 25;56(8):3177-90. |
||
| Additional Infomation | CXC Chemokine Receptor 2 Antagonist AZD5069 is an orally bioavailable, selective and reversible antagonist of CXC chemokine receptor 2 (CXCR2), with potential anti-inflammatory and antineoplastic activities. Upon administration, CXC chemokine receptor 2 antagonist AZD5069 directly binds to CXCR2 and inhibits its activation. This inhibits CXCR2-mediated signaling and may inhibit tumor cell proliferation in CXCR2-overexpressing tumor cells. In addition, AZD5069 reduces both neutrophil recruitment and migration from the systemic circulation into sites of inflammation, including the lung mucosa; it may also prevent neutrophil migration from the bone marrow. This results in the reduction of inflammation, mucus production, and neutrophil proteinase-mediated tissue destruction in the lung. CXCR2, a G protein-coupled receptor protein also known as IL-8 receptor B (IL-8RB), is upregulated in a variety of tumor cell types and plays a key role in tumor cell proliferation and progression; it is known to be elevated in several inflammatory diseases, such as chronic obstructive pulmonary disease (COPD), asthma and fibrotic pulmonary disorders. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.62 mg/mL (5.50 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.25 mg/mL (4.72 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 3: ≥ 2.25 mg/mL (4.72 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 4: ≥ 2.25 mg/mL (4.72 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 22.5 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. Solubility in Formulation 5: 5%DMSO + 40%PEG300 + 65%ddH2O: 8.0mg/ml (16.79mM) Solubility in Formulation 6: 12.5 mg/mL (26.23 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0986 mL | 10.4930 mL | 20.9859 mL | |
| 5 mM | 0.4197 mL | 2.0986 mL | 4.1972 mL | |
| 10 mM | 0.2099 mL | 1.0493 mL | 2.0986 mL |