Physicochemical Properties
| Molecular Formula | C32H40N8O5S |
| Molecular Weight | 648.7756 |
| Exact Mass | 648.28 |
| Elemental Analysis | C, 59.24; H, 6.21; N, 17.27; O, 12.33; S, 4.94 |
| CAS # | 2035509-96-5 |
| Related CAS # | 2035509-96-5 |
| PubChem CID | 122705989 |
| Appearance | Off-white to yellow solid powder |
| LogP | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 46 |
| Complexity | 1170 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | S(C([H])([H])[H])(N1C2=C([H])C([H])=C([H])C([H])=C2C(N(C([H])([H])[H])C2=C([H])N=C(N=C12)N([H])C1C([H])=C([H])C(=C([H])C=1OC([H])([H])C([H])([H])[H])C(N1C([H])([H])C([H])([H])C([H])(C([H])([H])C1([H])[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)=O)(=O)=O |
| InChi Key | HTFNVAVTYILUCF-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C32H40N8O5S/c1-5-45-28-20-22(30(41)39-14-12-23(13-15-39)38-18-16-36(2)17-19-38)10-11-25(28)34-32-33-21-27-29(35-32)40(46(4,43)44)26-9-7-6-8-24(26)31(42)37(27)3/h6-11,20-21,23H,5,12-19H2,1-4H3,(H,33,34,35) |
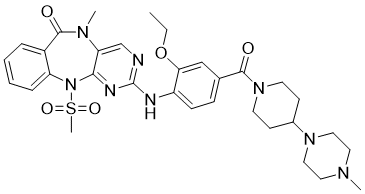
| Chemical Name | 2-[2-ethoxy-4-[4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl]anilino]-5-methyl-11-methylsulfonylpyrimido[4,5-b][1,4]benzodiazepin-6-one |
| Synonyms | AX15836; AX-15836; 2035509-96-5; AX15,836; AX-15,836; AX 15,836; 2-[2-ethoxy-4-[4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl]anilino]-5-methyl-11-methylsulfonylpyrimido[4,5-b][1,4]benzodiazepin-6-one; CHEMBL4541479; 2-((2-ethoxy-4-(4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl)phenyl)amino)-5-methyl-11-(methylsulfonyl)-5,11-dihydro-6H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6-one; 2-(2-Ethoxy-4-(4-(4-methylpiperazin-1-yl)piperidine-1-carbonyl)phenylamino)-5-methyl-11-(methylsulfonyl)-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one; AX 15836 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | ERK5 (IC50 = 8 nM) |
| ln Vitro | AX-15836 demonstrates ERK5 selectivity of more than 1,000-fold over a panel of more than 200 kinases. With a Kd of 3,600 nM, it also displays selectivity over BRD4. Peripheral blood mononuclear cells (PBMCs), endothelial cells, and oncogenic cell lines all exhibit a similar intracellular potency (4–9 nM) for AX15836. AX15836 was completely ineffective (EC50>10 M) at inhibiting the inflammatory cytokine response, indicating that the compounds' BRD inhibition component was what actually caused the cytokine reduction. Only a small number of genes exhibit differential expression in samples treated with AX15836 in HUVEC and HeLa cell types. In HeLa cells, AX15836 was able to demonstrably inhibit ERK5 that had been phosphorylated in response to EGF[1]. |
| Cell Assay |
For studies on cell proliferation, cells are exposed to DMSO vehicle (0.25% final volume) or an eight-point serial dilution series of AX-15836 (starting concentration of 15 μM). For MM.1S cells, compound was added 1 h before adding recombinant human IL-6 at 5 nM.. Utilizing the CellTiter-Glo 2.0 reagent, the relative number of viable cells was calculated 3 days later. The Synergy 2 multimode reader was used to read the Luminescence[1].
Preparation of cell samples for RNA-Seq. [1] Cells were preincubated with 0.1% DMSO vehicle, 1 μM AX-15836 (ERK5 inhibitor), 5 μM AX15839 (dual ERK5/BRD inhibitor), or 1 μM I-BET762 (BRD inhibitor) for 1 h. HUVECs and HeLa cells were then stimulated with their respective agonists (10 μg/mL Pam3CSK4 or 50 ng/mL EGF) for 5 h at 37 °C. Cells were processed to total RNA using the RNeasy kit. RNA was sent for RNA-Seq. One microgram total RNA from each sample was ribodepleted using the Ribo-Zero-rRNA Removal Kit. Sequencing libraries were then prepared from ribodepleted RNA using NEBNext Ultra RNA Library Prep Kit for Illumina following the manufacturer’s recommended protocol and barcoded using standard Illumina TruSeq barcoded adapter sequences. Final libraries were size-selected using Agencourt AMPure XP beads. Purified libraries were pooled and loaded onto an Illumina HiSeq2000 sequencer for 100-base single end sequencing with 7-base index reads. |
| Animal Protocol | Male CD-1 mice (∼12 wk old; Envigo) were used in this study. Animals were allowed to acclimate for a minimum of 48 h before the conduction of experiments. All animals were group housed in ventilated cages in the animal facility with regulated temperature from 20 °C to 26 °C and relative humidity at 30–70% with a 12/12-h day/night cycle. Pharmacokinetic studies were performed using fasted mice orally dosed at 50 mg/kg with AX-15836 formulated in 2.5% (vol/vol) dimethylacetamide: in 0.3% (wt/vol) carboxymethylcellulose. Blood was sampled for eight time points over 24 h. [1] |
| References |
[1]. ERK5 kinase activity is dispensable for cellular immune response and proliferation. Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11865-11870. |
| Additional Infomation | Unlike other members of the MAPK family, ERK5 contains a large C-terminal domain with transcriptional activation capability in addition to an N-terminal canonical kinase domain. Genetic deletion of ERK5 is embryonic lethal, and tissue-restricted deletions have profound effects on erythroid development, cardiac function, and neurogenesis. In addition, depletion of ERK5 is antiinflammatory and antitumorigenic. Small molecule inhibition of ERK5 has been shown to have promising activity in cell and animal models of inflammation and oncology. Here we report the synthesis and biological characterization of potent, selective ERK5 inhibitors. In contrast to both genetic depletion/deletion of ERK5 and inhibition with previously reported compounds, inhibition of the kinase with the most selective of the new inhibitors had no antiinflammatory or antiproliferative activity. The source of efficacy in previously reported ERK5 inhibitors is shown to be off-target activity on bromodomains, conserved protein modules involved in recognition of acetyl-lysine residues during transcriptional processes. It is likely that phenotypes reported from genetic deletion or depletion of ERK5 arise from removal of a noncatalytic function of ERK5. The newly reported inhibitors should be useful in determining which of the many reported phenotypes are due to kinase activity and delineate which can be pharmacologically targeted. [1] |
Solubility Data
| Solubility (In Vitro) | DMSO: ~100 mg/mL (~154.1 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (3.85 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5414 mL | 7.7068 mL | 15.4135 mL | |
| 5 mM | 0.3083 mL | 1.5414 mL | 3.0827 mL | |
| 10 mM | 0.1541 mL | 0.7707 mL | 1.5414 mL |