ATI-2341 (ATI2341), a pepducin targeting the C-X-C chemokine receptor type 4 (CXCR4), is a novel and selective allosteric agonist of CXCR4 with anti-inflammatory and anticancer activity. It causes the production of cAMP to be inhibited and calcium mobilization to be induced by activating the inhibitory heterotrimeric G protein (Gi). In CXCR4-HEK cells, ATI-2341 could dose-dependently block NKH477-induced cAMP accumulation, but it had no effect on HEK-293 parental cells that were naive. The ability of ATI-2341 to inhibit cAMP accumulation was totally eliminated when CXCR4-HEK cells were pretreated with pertussis toxin. In addition, ATI-2341 may cause a dose-dependent rise in intracellular calcium in wild-type CXCR4-transfected cells while having no effect on untransfected cells.
Physicochemical Properties
| Molecular Formula | C104H178N26O25S2 | |
| Molecular Weight | 2256.82 | |
| Exact Mass | 2255.289 | |
| CAS # | 1337878-62-2 | |
| Related CAS # | ATI-2341 TFA | |
| PubChem CID | 121513892 | |
| Appearance | Typically exists as solid at room temperature | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.625 | |
| LogP | 3.81 | |
| Hydrogen Bond Donor Count | 32 | |
| Hydrogen Bond Acceptor Count | 32 | |
| Rotatable Bond Count | 89 | |
| Heavy Atom Count | 157 | |
| Complexity | 4370 | |
| Defined Atom Stereocenter Count | 16 | |
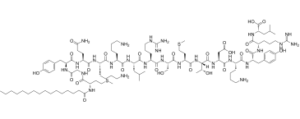
| SMILES | [C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(=O)O)CC(C)C)(NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)NC(=O)[C@]([H])([C@H](O)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCSC)NC(=O)CCCCCCCCCCCCCCC)CC1C=CC(O)=CC=1)CC1C=CC(O)=CC=1 |
|
| InChi Key | YMLOFEANRZSEGQ-QNHYGVTPSA-N | |
| InChi Code | InChI=1S/C104H178N26O25S2/c1-9-10-11-12-13-14-15-16-17-18-19-20-21-35-84(136)116-75(46-53-156-7)88(140)115-60-85(137)117-78(57-65-36-40-67(133)41-37-65)97(149)123-74(44-45-83(108)135)94(146)119-69(30-22-25-48-105)89(141)118-70(31-23-26-49-106)90(142)125-77(55-62(2)3)96(148)121-73(34-29-52-114-104(111)112)93(145)129-82(61-131)100(152)124-76(47-54-157-8)95(147)130-87(64(6)132)101(153)127-80(59-86(138)139)99(151)120-71(32-24-27-50-107)91(143)126-79(58-66-38-42-68(134)43-39-66)98(150)122-72(33-28-51-113-103(109)110)92(144)128-81(102(154)155)56-63(4)5/h36-43,62-64,69-82,87,131-134H,9-35,44-61,105-107H2,1-8H3,(H2,108,135)(H,115,140)(H,116,136)(H,117,137)(H,118,141)(H,119,146)(H,120,151)(H,121,148)(H,122,150)(H,123,149)(H,124,152)(H,125,142)(H,126,143)(H,127,153)(H,128,144)(H,129,145)(H,130,147)(H,138,139)(H,154,155)(H4,109,110,113)(H4,111,112,114)/t64-,69+,70+,71+,72+,73+,74+,75+,76+,77+,78+,79+,80+,81+,82+,87+/m1/s1 | |
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[2-[[(2S)-2-(hexadecanoylamino)-4-methylsulfanylbutanoyl]amino]acetyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-oxopentanoyl]amino]hexanoyl]amino]hexanoyl]amino]-4-methylpentanoyl]amino]-5-carbamimidamidopentanoyl]amino]-3-hydroxypropanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-hydroxybutanoyl]amino]-3-carboxypropanoyl]amino]hexanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]-4-methylpentanoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CXCR4 ( EC50 = 194 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | ATI-2341 is a potent and functionally selective allosteric agonist of C-X-C chemokine receptor type 4 (CXCR4), acting as a biased ligand that prefers Gαi activation over Gα13. ATI-2341 activates the inhibitory heterotrimeric G protein (Gi) to encourage the inhibition of cAMP production and to cause calcium mobilization. | ||
| Cell Assay | CXCR4-eGFP receptors are transiently transfected into HEK-293 cells, and 24 hours after transfection, the cells are plated on poly-D-lysine-coated glass coverslips. The cells are treated with either vehicle alone or different concentrations of ATI-2341 for 30 minutes at 37 degrees Celsius the following day. They are then fixed with methanol for 5 minutes at -20 degrees Celsius. An inverted Zeiss Axiovert microscope is used to directly visualize GFP fluorescence. Adobe Illustrator and Photoshop are used for image processing. | ||
| Animal Protocol |
|
||
| References |
[1]. Proc Natl Acad Sci U S A . 2010 Dec 21;107(51):22255-9. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.4431 mL | 2.2155 mL | 4.4310 mL | |
| 5 mM | 0.0886 mL | 0.4431 mL | 0.8862 mL | |
| 10 mM | 0.0443 mL | 0.2216 mL | 0.4431 mL |