Physicochemical Properties
| Molecular Formula | C29H31CLFN5O5 |
| Molecular Weight | 584.0454 |
| Exact Mass | 583.2 |
| Elemental Analysis | C, 59.64; H, 5.35; Cl, 6.07; F, 3.25; N, 11.99; O, 13.70 |
| CAS # | 2095719-92-7 |
| Related CAS # | 2095719-92-7 |
| PubChem CID | 129053037 |
| Appearance | White to off-white solid powder |
| LogP | 2.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Heavy Atom Count | 41 |
| Complexity | 900 |
| Defined Atom Stereocenter Count | 2 |
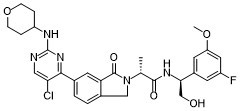
| SMILES | C[C@H](C(=O)N[C@H](CO)C1=CC(=CC(=C1)F)OC)N2CC3=C(C2=O)C=C(C=C3)C4=NC(=NC=C4Cl)NC5CCOCC5 |
| InChi Key | BVRGQPJKSKKGIH-PUAOIOHZSA-N |
| InChi Code | InChI=1S/C29H31ClFN5O5/c1-16(27(38)34-25(15-37)19-9-20(31)12-22(10-19)40-2)36-14-18-4-3-17(11-23(18)28(36)39)26-24(30)13-32-29(35-26)33-21-5-7-41-8-6-21/h3-4,9-13,16,21,25,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t16-,25-/m1/s1 |
| Chemical Name | (2R)-2-[5-[5-chloro-2-(oxan-4-ylamino)pyrimidin-4-yl]-3-oxo-1H-isoindol-2-yl]-N-[(1S)-1-(3-fluoro-5-methoxyphenyl)-2-hydroxyethyl]propanamide |
| Synonyms | ASTX-029; ASTX029; ASTX 029 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | ERK1; ERK2 |
| ln Vitro | ASTX029 (96 h) has IC50 values ranging from 1.8 to 380 nM, which means that it suppresses the phosphorylation of human cancer cells with mutations that activate MAPK [2]. With IC50 values of 3.3 for A375 and HCT116 cells, respectively, ASTX029 (2 h) suppresses RSK phosphorylation. In A375 and HCT116 cells, ASTX029 (10 and 100 nM, 0-72 h) causes cell life cycle candles in the G1 phase and induces transplanting [2]. |
| ln Vivo | In tumor-bearing Colo205 (BRAFV600E-mottled colorectal cancer) tumors, ASTX029 (20–75 mg/kg, neck) inhibits tumor growth [2]. T1/2 was 2.9 hours, F (%) was 42%, and AUC for ASTX029 (5 mg/kg, neck, neck) was 1600 ng h/mL [3]. |
| Cell Assay |
Western Blot Analysis[2] Cell Types: A375 and HCT116 Cell Tested Concentrations: 1 nM-100 nM Incubation Duration: 2 hrs (hours) Experimental Results: pRSK and pERK diminished. Apoptosis analysis[2] Cell Types: A375 and HCT116 Cell Tested Concentrations: 0-100 nM Incubation Duration: 0-72 hrs (hours) Experimental Results: Cell arrest in G1 phase. Cleaved PARP and Bim protein levels increased. |
| Animal Protocol |
Animal/Disease Models: Colo205, A375, Calu-6, HCC44, HCT116 or MA-MEL-28 xenograft tumors in mice [2] Doses: 75 mg/kg Route of Administration: po (po (oral gavage)) one time/day Experimental Results: In multiple tumor models Inhibit tumor growth. |
| References |
[1]. 6-pyrimidin-isoindole derivative as erk1/2 inhibitor. WO2018193410A1. [2]. ASTX029, a Novel Dual-mechanism ERK Inhibitor, Modulates Both the Phosphorylation and Catalytic Activity of ERK. Mol Cancer Ther. 2021 Oct;20(10):1757-1768. |
| Additional Infomation | Beroterkib Anhydrous is the anhydrous form of beroterkib, an orally bioavailable inhibitor of the extracellular signal-regulated kinases (ERK) 1 and 2, with potential antineoplastic activity. Upon administration, beroterkib specifically binds to and inhibits both ERK 1 and 2, thereby preventing the activation of mitogen-activated protein kinase (MAPK)/ERK-mediated signal transduction pathways. This results in the inhibition of ERK-dependent tumor cell proliferation and survival. The MAPK/ERK pathway is often upregulated in a variety of tumor cell types and plays a key role in the proliferation, differentiation and survival of tumor cells. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 100~250 mg/mL (171.2~428.1 mM) Ethanol: ~50 mg/mL (~85.6 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.56 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (3.56 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.56 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7122 mL | 8.5609 mL | 17.1218 mL | |
| 5 mM | 0.3424 mL | 1.7122 mL | 3.4244 mL | |
| 10 mM | 0.1712 mL | 0.8561 mL | 1.7122 mL |