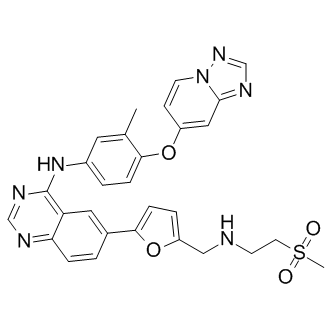
ARRY-380 analog, an analog of ARRY-380 (Irbinitinib, formerly known as ARRY-380 and ONT-380 or Tucatinib) which is a potent and selective small molecule inhibitor of HER2 with IC50 value of 8 nM, it is equally potent against truncated p95-HER2, and is 500-fold more selective for HER2 versus EGFR. Irbinitinib acts by blocking the proliferation and phosphorylation of HER2 and its downstream effector, Akt. By contrast, in the EGFR overexpressing cell lines, it weakly inhibits phosphorylation and proliferation, demonstrating that Irbinitinib may have potential to block HER2 signaling without causing the toxicities of EGFR inhibition. Therefore, it has the potential to be used as an anticancer agent.
Physicochemical Properties
| Molecular Formula | C29H27N7O4S | |
| Molecular Weight | 569.63 | |
| Exact Mass | 569.184 | |
| Elemental Analysis | C, 61.15; H, 4.78; N, 17.21; O, 11.23; S, 5.63 | |
| CAS # | 937265-83-3 | |
| Related CAS # | 937263-43-9;937265-83-3 (ARRY-380 analog); | |
| PubChem CID | 42598643 | |
| Appearance | Light brown to brown solid | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.709 | |
| LogP | 3.59 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 10 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 41 | |
| Complexity | 954 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O=S(CCNCC1=CC=C(C2C=C3C(N=CN=C3NC3C=C(C)C(OC4=CC5N(N=CN=5)C=C4)=CC=3)=CC=2)O1)(C)=O |
|
| InChi Key | QVMNYGOVNWWFKF-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C29H27N7O4S/c1-19-13-21(4-7-26(19)39-22-9-11-36-28(15-22)32-18-34-36)35-29-24-14-20(3-6-25(24)31-17-33-29)27-8-5-23(40-27)16-30-10-12-41(2,37)38/h3-9,11,13-15,17-18,30H,10,12,16H2,1-2H3,(H,31,33,35) | |
| Chemical Name | 6-[5-[[[2-(Methylsulfonyl)ethyl]amino]methyl]-2-furanyl]-N-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-4-quinazolinamine | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets |
EGFR (ErbB1); Mcl-1:ARRY-380 analog (Compound 249) is a selective small molecule inhibitor of Mcl-1 with an IC₅₀ of 25 nM in enzyme binding assays. [1] |
||
| ln Vitro |
- Mcl-1 binding and apoptosis induction:In biochemical assays, Compound 249 (10–100 nM) disrupts Mcl-1 interaction with pro-apoptotic proteins (e.g., Bak) in pull-down assays. In Mcl-1-dependent cancer cell lines (e.g., MOLM-13), it induces apoptosis (50–70% caspase-3 activation) at EC₅₀ of 50 nM, as measured by flow cytometry. [1] - Antiproliferative activity:Against Mcl-1-overexpressing leukemia cells (MV4-11), Compound 249 inhibits proliferation with an IC₅₀ of 80 nM in MTT assays. Minimal activity is observed in Mcl-1-low cells (e.g., HL-60, IC₅₀ > 1,000 nM). [1] In vitro activity: ARRY-380 analog, an analog of ARRY-380 (Irbinitinib, formerly known as ARRY-380 and ONT-380 or Tucatinib) which is a potent and selective small molecule inhibitor of HER2 with IC50 value of 8 nM, it is equally potent against truncated p95-HER2, and is 500-fold more selective for HER2 versus EGFR. Irbinitinib acts by blocking the proliferation and phosphorylation of HER2 and its downstream effector, Akt. By contrast, in the EGFR overexpressing cell lines, it weakly inhibits phosphorylation and proliferation, demonstrating that Irbinitinib may have potential to block HER2 signaling without causing the toxicities of EGFR inhibition. Therefore, it has the potential to be used as an anticancer agent. Kinase Assay: Irbinitinib, formerly known as ARRY-380 and ONT-380 or Tucatinib, is a potent and selective small molecule inhibitor of HER2 with IC50 value of 8 nM, it is equally potent against truncated p95-HER2, and is 500-fold more selective for HER2 versus EGFR. Irbinitinib acts by blocking the proliferation and phosphorylation of HER2 and its downstream effector, Akt. By contrast, in the EGFR overexpressing cell lines, it weakly inhibits phosphorylation and proliferation, demonstrating that Irbinitinib may have potential to block HER2 signaling without causing the toxicities of EGFR inhibition. Therefore, it has the potential to be used as an anticancer agent. Cell Assay: ONT-380 has nanomolar activity against purified HER2 enzyme and is approximately 500-fold selective for HER2 versus EGFR in cell-based assays. In the EGFR overexpressing cell lines, it weakly inhibits phosphorylation and proliferation, demonstrating that Irbinitinib may have potential to block HER2 signaling without causing the toxicities of EGFR inhibition. |
||
| ln Vivo |
|
||
| Enzyme Assay |
Mcl-1 binding assay:
1. Recombinant Mcl-1 protein (1 μM) is incubated with biotinylated Bak BH3 peptide (50 nM) and Compound 249 (0.1–1,000 nM) in binding buffer.
2. After 1 hour at 25°C, streptavidin beads are added to capture the complex.
3. Bound Mcl-1 is detected by ELISA, and IC₅₀ is calculated as the concentration causing 50% inhibition of Mcl-1-Bak interaction. [1] |
||
| Cell Assay |
Apoptosis and proliferation assay:
1. MOLM-13 cells (5×10⁴ cells/well) are treated with Compound 249 (10–1,000 nM) for 24 hours.
2. Apoptosis is assessed by Annexin V/PI staining and flow cytometry; caspase-3 activation is confirmed by Western blot.
3. Proliferation is measured by MTT reduction, with IC₅₀ determined as the concentration reducing absorbance by 50%. [1] |
||
| Animal Protocol |
|
||
| References | [1]. Small molecule inhibitors of mcl-1 and uses thereof. WO2015153959A2. | ||
| Additional Infomation |
- Mechanism of action:Compound 249 competitively binds to the BH3-binding groove of Mcl-1, displacing pro-apoptotic proteins and triggering mitochondrial outer membrane permeabilization (MOMP). This leads to caspase activation and apoptosis in Mcl-1-dependent cancers. [1] - Therapeutic potential:Investigated for hematologic malignancies (e.g., acute myeloid leukemia) where Mcl-1 overexpression confers resistance to standard therapies. [1] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.65 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.08 mg/mL (3.65 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7555 mL | 8.7776 mL | 17.5553 mL | |
| 5 mM | 0.3511 mL | 1.7555 mL | 3.5111 mL | |
| 10 mM | 0.1756 mL | 0.8778 mL | 1.7555 mL |