Physicochemical Properties
| Exact Mass | 403.12 |
| CAS # | 330461-64-8 |
| PubChem CID | 1341463 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Index of Refraction | 1.630 |
| LogP | 4.11 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 28 |
| Complexity | 650 |
| Defined Atom Stereocenter Count | 0 |
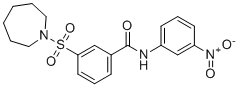
| SMILES | S(C1=C([H])C([H])=C([H])C(C(N([H])C2C([H])=C([H])C([H])=C(C=2[H])[N+](=O)[O-])=O)=C1[H])(N1C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C1([H])[H])(=O)=O |
| InChi Key | HAYBKCHPEBZNGW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C19H21N3O5S/c23-19(20-16-8-6-9-17(14-16)22(24)25)15-7-5-10-18(13-15)28(26,27)21-11-3-1-2-4-12-21/h5-10,13-14H,1-4,11-12H2,(H,20,23) |
| Chemical Name | 3-(azepan-1-ylsulfonyl)-N-(3-nitrophenyl)benzamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | On Drosophila with Huntington's disease, AK-1 at 10 μM had a strong neuroprotective effect, increasing the number of striated muscles from 5.2 to 5.6 [1]. With an IC50 of 12.5 μM, AK-1 is a powerful, selective, and cell-permeable inhibitor of SIRT2 [2]. By blocking the NF-κB/CSN2 pathway, AK-1 therapy causes the Snail transcription factor to be broken down by proteases. Decreased snail levels cause p21 to be overexpressed, which slows down wound healing, G1 arrest, and proliferation. In HT-29 colon cancer cells, AK-1 also regulates the Snail-p21 axis [3]. In hypoxic environments, AK-1 enhances HIF-1α's ubiquitination in a VHL-dependent manner, which triggers HIF-1α's destruction via the proteasomal route. Downregulating HIF-1α expression in AK-1-treated cells lowers its transcriptional activity, which in turn lowers the expression of BNIP3, one of the HIF-1 target genes [4]. |
| References |
[1]. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7927-32. [2]. AK-1, a SIRT2 inhibitor, destabilizes HIF-1α and diminishes its transcriptional activity during hypoxia. Cancer Lett. 2016 Apr 1;373(1):138-45. [3]. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7927-32. [4]. A Brain-Permeable Small Molecule Reduces Neuronal Cholesterol by Inhibiting Activity of Sirtuin 2 Deacetylase. ACS Chem Biol. 2011 Jun 17;6(6):540-6. [5]. AK-1, a specific SIRT2 inhibitor, induces cell cycle arrest by downregulating Snail in HCT116 human colon carcinoma cells. Cancer Lett. 2015 Jan 28;356(2 Pt B):637-45. |
Solubility Data
| Solubility (In Vitro) | DMSO : ≥ 50 mg/mL (~123.93 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.20 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |