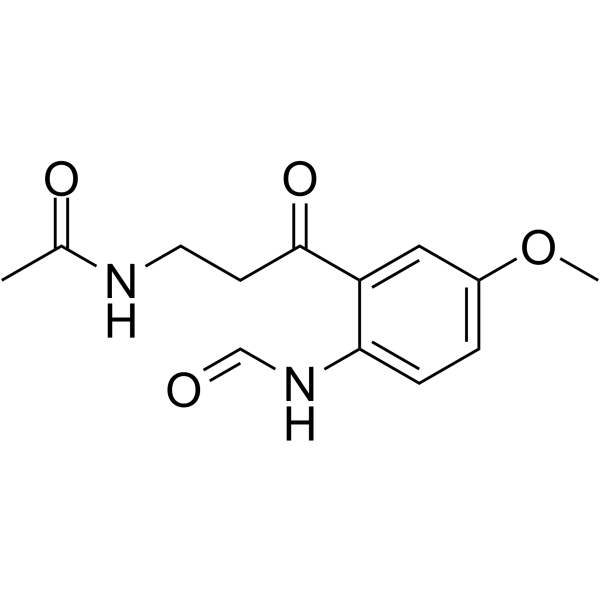
Physicochemical Properties
| Molecular Formula | C13H16N2O4 |
| Molecular Weight | 264.28 |
| Exact Mass | 264.11 |
| CAS # | 52450-38-1 |
| PubChem CID | 171161 |
| Appearance | Light yellow to light brown solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 589.4±50.0 °C at 760 mmHg |
| Melting Point | 138-140ºC |
| Flash Point | 310.3±30.1 °C |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.560 |
| LogP | 0.82 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 19 |
| Complexity | 333 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | JYWNYMJKURVPFH-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C13H16N2O4/c1-9(17)14-6-5-13(18)11-7-10(19-2)3-4-12(11)15-8-16/h3-4,7-8H,5-6H2,1-2H3,(H,14,17)(H,15,16) |
| Chemical Name | N-[3-(2-formamido-5-methoxyphenyl)-3-oxopropyl]acetamide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Human Endogenous Metabolite |
| ln Vitro | One of the metabolites of melatonin, AFMK is produced by a variety of metabolic routes, including nonenzymatic, pseudoenzymatic, and enzymatic ones[1]. Pretreatment with AFMK dramatically reduces DNA damage. The electron spin resonance (ESR) investigation used to test AFMK's in vitro hydroxyl radical scavenging potential revealed an extremely high level of activity. The ESR study yielded IC50 values of 338.08 nM. AFMK, a metabolite of melatonin, is a biogenic amine that is rarely studied[2]. In comparison to the outcomes achieved with Gemcitabine alone, AFMK given to PANC-1 in combination with Gemcitabine suppresses the synthesis of HSP70 and cIAP-2[3]. |
| ln Vivo | In vivo, AFMK is a strong antioxidant. AFMK dramatically reverses the decrease in mice's plasma's overall antioxidant capacity that is brought on by radiation[2]. |
| Cell Assay |
Western Blot Analysis[3] Cell Types: Human pancreatic carcinoma cell line (PANC-1) Tested Concentrations: 0.001, 0.1, 10, 1000 nM Incubation Duration: Experimental Results: Augmented the inhibitory effects on HSP70 expression from 0.47 (Gemcitabine alone) to 0.13 (10 nM AFMK), 0.08 (0.1 nM AFMK) and 0.01 (0.001 nM AFMK). |
| Animal Protocol |
Animal/Disease Models: Male C57BL mice 8 wk of age[2] Doses: 10 mg/kg body weight Route of Administration: intraperitoneal (ip) injection Experimental Results: Radiation-induced decline in the total antioxidant capacity of plasma was Dramatically reversed in AFMK pretreated mice. AFMK-pretreated irradiated groups demonstrated a Dramatically lower value of comet tail length and % DNA in tail. |
| References |
[1]. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res. 2013 Apr;54(3):245-57. [2]. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res. 2007 Apr;42(4):386-93. [3]. Melatonin and its metabolite N1-acetyl-N2-formyl-5-methoxykynuramine (afmk) enhance chemosensitivity to gemcitabine in pancreatic carcinoma cells (PANC-1). Pharmacol Rep. 2018 Dec;70(6):1079-1088. |
| Additional Infomation | N-gamma-Acetyl-N-2-Formyl-5-Methoxykynurenamine is an aromatic ketone. |
Solubility Data
| Solubility (In Vitro) | DMSO: 50 mg/mL (189.19 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.46 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.46 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.46 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7839 mL | 18.9193 mL | 37.8387 mL | |
| 5 mM | 0.7568 mL | 3.7839 mL | 7.5677 mL | |
| 10 mM | 0.3784 mL | 1.8919 mL | 3.7839 mL |