ACY-738 (ACY738) is a novel, potent, selective, brain penetrable and orally-bioavailable HDAC6 inhibitor with neuroprotective and anticancer activities. It also inhibits HDAC1, HDAC2, and HDAC3, with IC50s of 94, 128, and 218 nM; its IC50 for HDAC6 is 1.7 nM. In RN46A-B14 cells, ACY-738 (2.5 μM) raises the acetylated (lysine 40) fraction of α-tubulin. Cell death induced by ACY-738 (10 μM) is similar to that of FK228 and LBH589. ACY-738 has a selectivity of 60–1500 times greater than class I HDACs and low nanomolar potency when inhibiting HDAC6. ACY-738 stimulates mouse exploratory behaviors in unfamiliar but novel environments and causes dramatic increases in α-tubulin acetylation in the brain, unlike tubastatin A, a reference HDAC6 inhibitor with similar potency and peripheral activity but less brain bioavailability.
Physicochemical Properties
| Molecular Formula | C14H14N4O2 | |
| Molecular Weight | 270.29 | |
| Exact Mass | 270.111 | |
| Elemental Analysis | C, 62.21; H, 5.22; N, 20.73; O, 11.84 | |
| CAS # | 1375465-91-0 | |
| Related CAS # |
|
|
| PubChem CID | 57381425 | |
| Appearance | White to off-white solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Index of Refraction | 1.715 | |
| LogP | 0.24 | |
| Hydrogen Bond Donor Count | 3 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 4 | |
| Heavy Atom Count | 20 | |
| Complexity | 345 | |
| Defined Atom Stereocenter Count | 0 | |
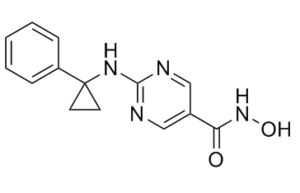
| SMILES | O=C(C1=C([H])N=C(N=C1[H])N([H])C1(C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H])N([H])O[H] |
|
| InChi Key | LIIWIMDSZVNYHY-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C14H14N4O2/c19-12(18-20)10-8-15-13(16-9-10)17-14(6-7-14)11-4-2-1-3-5-11/h1-5,8-9,20H,6-7H2,(H,18,19)(H,15,16,17) | |
| Chemical Name | N-hydroxy-2-[(1-phenylcyclopropyl)amino]pyrimidine-5-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HDAC6 ( IC50 = 1.7 nM ); HDAC1 ( IC50 = 94 nM ); HDAC2 ( IC50 = 128 nM ); HDAC3 ( IC50 = 218 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | ACY-738 is a newly developed HDAC6 inhibitor that is highly selective, potent, and orally bioavailable. Its IC50 is 1.7 nM, and it also inhibits HDAC1, HDAC2, and HDAC3 at IC50s of 94, 128, and 218 nM. | ||
| Cell Assay | In RN46A-B14 cells, ACY-738 (2.5 μM) raises the acetylated (lysine 40) fraction of α-tubulin. | ||
| Animal Protocol |
|
||
| References |
[1]. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014 Jan;39(2):389-400. [2]. Specific HDAC6 inhibition by ACY-738 reduces SLE pathogenesis in NZB/W mice. Clin Immunol. 2016 Jan;162:58-73. [3]. Histone deacetylase (HDAC) inhibitors as single agents induce multiple myeloma cell death principally through the inhibition of class I HDAC. Br J Haematol. 2013 Aug;162(4):559-62. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (7.70 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (7.70 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (7.70 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6997 mL | 18.4986 mL | 36.9973 mL | |
| 5 mM | 0.7399 mL | 3.6997 mL | 7.3995 mL | |
| 10 mM | 0.3700 mL | 1.8499 mL | 3.6997 mL |