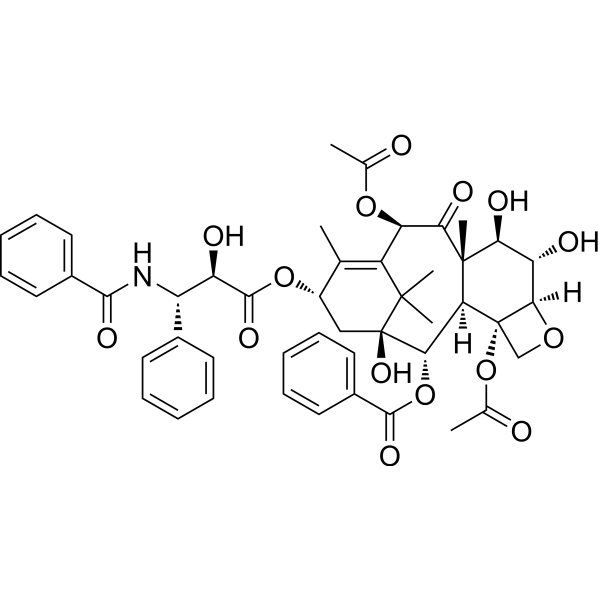
Physicochemical Properties
| Molecular Formula | C47H51NO15 |
| Exact Mass | 869.325 |
| CAS # | 153212-75-0 |
| PubChem CID | 10056458 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 979.8±65.0 °C at 760 mmHg |
| Melting Point | 177-180ºC |
| Flash Point | 546.4±34.3 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.646 |
| LogP | 7.32 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 63 |
| Complexity | 1830 |
| Defined Atom Stereocenter Count | 12 |
| SMILES | CC1=C2[C@@H](OC(C)=O)C([C@]3([C@@H]([C@]4(OC(C)=O)CO[C@@H]4[C@@H](O)[C@@H]3O)[C@H](OC(C5=CC=CC=C5)=O)[C@](C2(C)C)(O)C[C@@H]1OC([C@H](O)[C@@H](NC(C6=CC=CC=C6)=O)C7=CC=CC=C7)=O)C)=O |
| InChi Key | NDCWHEDPSFRTDA-FJMWQILYSA-N |
| InChi Code | InChI=1S/C47H51NO15/c1-24-30(61-43(57)33(51)32(27-16-10-7-11-17-27)48-41(55)28-18-12-8-13-19-28)22-47(58)40(62-42(56)29-20-14-9-15-21-29)36-45(6,38(54)35(60-25(2)49)31(24)44(47,4)5)37(53)34(52)39-46(36,23-59-39)63-26(3)50/h7-21,30,32-37,39-40,51-53,58H,22-23H2,1-6H3,(H,48,55)/t30-,32-,33+,34-,35+,36-,37-,39+,40-,45-,46+,47+/m0/s1 |
| Chemical Name | [(1S,2S,3R,4S,7R,8S,9R,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,8,9-trihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate |
| Synonyms | 6alpha-hydroxypaclitaxel; 6-Hydroxytaxol; 6alpha-Hydroxy Paclitaxel; 6-Hydroxypaclitaxel; 6; A-Hydroxy Paclitaxel; 6alpha-Hydroxytaxol; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Paclitaxel metabolite |
| ln Vitro | Paclitaxel has been considered to cause OATP1B-mediated drug-drug interactions at therapeutic doses; however, its clinical relevance has not been demonstrated. This study aimed to elucidate in vivo inhibition potency of paclitaxel against OATP1B1 and OATP1B3 using endogenous OATP1B biomarkers. Paclitaxel is an inhibitor of OATP1B1 and OATP1B3, with Ki of 0.579 ± 0.107 and 5.29 ± 3.87 μM, respectively. Preincubation potentiated its inhibitory effect on both OATP1B1 and OATP1B3, with Ki of 0.154 ± 0.031 and 0.624 ± 0.183 μM, respectively. Ten patients with non-small cell lung cancer who received 200 mg/m2 of paclitaxel by a 3-hour infusion were recruited. Plasma concentrations of 10 endogenous OATP1B biomarkers-namely, coproporphyrin I, coproporphyrin III, glycochenodeoxycholate-3-sulfate, glycochenodeoxycholate-3-glucuronide, glycodeoxycholate-3-sulfate, glycodeoxycholate-3-glucuronide, lithocholate-3-sulfate, glycolithocholate-3-sulfate, taurolithocholate-3-sulfate, and chenodeoxycholate-24-glucuronide-were determined in the patients with non-small cell lung cancer on the day before paclitaxel administration and after the end of paclitaxel infusion for 7 hours. Paclitaxel increased the area under the plasma concentration-time curve (AUC) of the endogenous biomarkers 2- to 4-fold, although a few patients did not show any increment in the AUC ratios of lithocholate-3-sulfate, glycolithocholate-3-sulfate, and taurolithocholate-3-sulfate. Therapeutic doses of paclitaxel for the treatment of non-small cell lung cancer (200 mg/m2) will cause significant OATP1B1 inhibition during and at the end of the infusion. This is the first demonstration that endogenous OATP1B biomarkers could serve as surrogate biomarkers in patients. SIGNIFICANCE STATEMENT: Endogenous biomarkers can address practical and ethical issues in elucidating transporter-mediated drug-drug interaction (DDI) risks of anticancer drugs clinically. We could elucidate a significant increment of the plasma concentrations of endogenous OATP1B biomarkers after a 3-hour infusion (200 mg/m2) of paclitaxel, a time-dependent inhibitor of OATP1B, in patients with non-small cell lung cancer. The endogenous OATP1B biomarkers are useful to assess the possibility of OATP1B-mediated DDIs in patients and help in appropriately designing a dosing schedule to avoid the DDIs[1]. |
| Cell Assay |
In Vitro Transport Study Using Transporter-Expressing Cells.[1] HEK293 cells, which were stably transfected with OATP1B1, OATP1B3, OAT1, OAT3, or empty vector, were established previously (Deguchi et al., 2004; Hirano et al., 2004). The transport study was performed according to the previous reports (Deguchi et al., 2004; Hirano et al., 2004). Briefly, HEK293 cells were seeded at 2 × 105 cells per well in a 24-well plate coated with poly-l-lysine (50 mg/l) and poly-l-ornithine (50 mg/l). After 48 hours, the medium was substituted by Dulbecco’s modified Eagle’s medium containing 5 mM sodium butyrate. After 24 hours, the transport study was conducted. Cells were washed twice and preincubated with Krebs-Henseleit (KH) buffer at 37°C for 5 minutes. Then, substrates were added to initiate the uptake. At the designated times, incubation buffer was removed, and ice-cold KH buffer was added to terminate the uptake. To evaluate the inhibition potency of paclitaxel and 6α-hydroxy paclitaxel, either paclitaxel or 6α-hydroxy paclitaxel was added simultaneously with the test substrate. When the time dependence of the inhibitory effect was assessed (shown in Fig. 1 and Supplemental Fig. 8), cells were preincubated in the presence of paclitaxel or 6α-hydroxy paclitaxel for 15 minutes. In a separate experiment (shown in Supplemental Fig. 6A), cells were preincubated in KH buffer containing paclitaxel for the designated periods (5, 10, 30, 60 minutes) and then washed. After washing, uptake was initiated in the absence of inhibitor. To assess the duration of the inhibitory effect (shown in Supplemental Fig. 6B), cells were preincubated with KH buffer containing paclitaxel for 30 minutes and incubated with paclitaxel-free KH buffer for the designated periods (0, 10, 30, 60 minutes). Then, uptake was initiated in the absence of paclitaxel. Western Blotting to Detect OATP1B1 and OATP1B3 in Whole Cells and Cell Surface.[1] Cells were harvested by cell scraper and collected by centrifugation (12,000g, 5 minutes, 4°C). They were lysed by cell lysis buffer. The samples were centrifuged at 12,000g for 10 minutes at 4°C, and then the supernatants were used in the analysis. The 4× SDS sample buffer (12% SDS, 25% glycerol, 150 mM Tris-HCl pH 7.0, 0.05% bromophenol blue, 6% β-mercaptoethanol) and 2-mercaptoethanol were added 1/4 and 1/30 volume of the sample, respectively, and heated at 60°C for 5 minutes. Then, the whole-cell samples were subjected to Western blot analysis. |
| References |
[1]. Alteration in the Plasma Concentrations of Endogenous Organic Anion-Transporting Polypeptide 1B Biomarkers in Patients with Non-Small Cell Lung Cancer Treated with Paclitaxel. Drug Metab Dispos. 2020 May;48(5):387-394. |
| Additional Infomation |
6-hydroxypaclitaxel is a taxane diterpenoid that consists of paclitaxel bearing an additional hydroxy substituent at the 6alpha-position. It has a role as an antineoplastic agent. It is a taxane diterpenoid and a tetracyclic diterpenoid. It is functionally related to a paclitaxel. 6alpha-Hydroxypaclitaxel has been reported in Homo sapiens with data available. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |