Physicochemical Properties
| Molecular Formula | C11H16CLNO |
| Molecular Weight | 213.70 |
| Exact Mass | 213.092 |
| CAS # | 3880-76-0 |
| PubChem CID | 12924837 |
| Appearance | Off-white to gray solid powder |
| Boiling Point | 341.2ºC at 760mmHg |
| Flash Point | 160.2ºC |
| LogP | 3.013 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 14 |
| Complexity | 172 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | GDFOHXLSNDZEHH-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C11H15NO.ClH/c1-13-11-4-2-3-8-5-6-9(12)7-10(8)11;/h2-4,9H,5-7,12H2,1H3;1H |
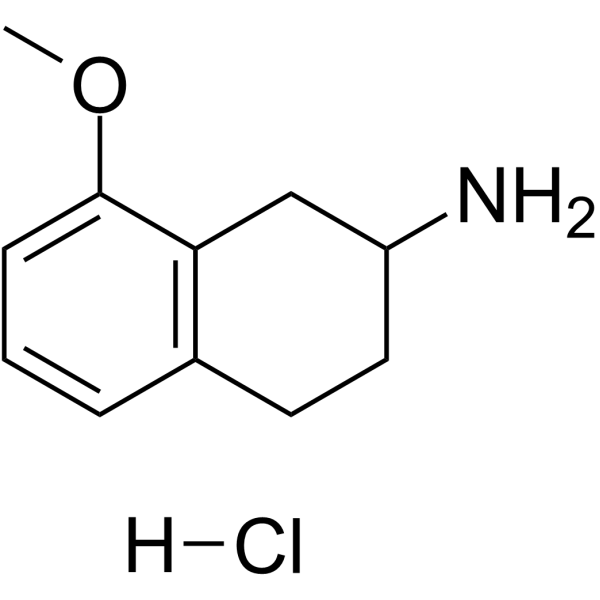
| Chemical Name | 8-methoxy-1,2,3,4-tetrahydronaphthalen-2-amine;hydrochloride |
| Synonyms | 3880-76-0; 8-methoxy-1,2,3,4-tetrahydronaphthalen-2-amine hydrochloride; 2-amino-8-methoxy-1,2,3,4-tetrahydronaphthalene hcl; 5-HT1A modulator 2 hydrochloride; (S)-(-)-8-methoxy-2-aminotetraline hydrochloride; (R)-(+)-8-Methoxy-2-aminotetraline hydrochloride; 5-HT1A modulator 2 (hydrochloride); 8-methoxy-2-aminotetralin hydrochloride; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Ki: 53 nM (5-HT1A)[1] |
| ln Vitro | 5-HT1A modulator 2 hydrochloride (compound 3) binds to 5-HT1A with a 53 nM Ki[1]. |
| References |
[1]. 2-(Alkylamino)tetralin derivatives: interaction with 5-HT1A serotonin binding sites. Journal of Medicinal Chemistry, 1989;32(1), 253–256. |
| Additional Infomation | 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) is a selective 5-HT1A serotonin agonist. Derivatives of 8-OH-DPAT with amine substituents larger or more bulky than n-propyl appear to be inactive in a presynaptic biochemical assay measuring agonist-induced feedback inhibition of 5-HT synthesis but have never been examined in brain binding assays. A series of N-phenylalkyl derivatives of 8-methoxy-2-aminotetralin was evaluated at [3H]-8-OH-DPAT-labeled 5-HT1A sites in rat brain hippocampal membranes. All of the phenylalkyl derivatives displayed significant affinity for these sites and, of the agents examined, the 3-phenylpropyl 8-hydroxy analogue appears to be optimal and had an affinity (Ki = 1.9 nM) comparable to that of 8-OH-DPAT (Ki = 1.2 nM). In addition, the presence of an oxygen-containing substituent at the 8-position of the tetralin ring is not necessary for good affinity, and secondary amines and tertiary amines displayed equal affinity at central 5-HT1A binding sites. 5-HT1A sites are found both pre- and postsynaptically; thus, differences observed in the biochemical assay as compared to the results of the present binding study could be due to different structural requirements of these two receptors. This seems unlikely, however, because there was little difference in the affinities of several selected analogues for striatal versus hippocampal binding sites. Because we have now demonstrated that amine substituents larger than propyl, and an unsubstituted 8-position, are well tolerated by central 5-HT1A sites, future studies aimed at the development of new serotonergic tetralin analogues need not be limited to N-propyl or 8-hydroxy derivatives of 2-aminotetralin. [1] |
Solubility Data
| Solubility (In Vitro) | DMSO: 100 mg/mL (467.95 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (11.70 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (11.70 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.6795 mL | 23.3973 mL | 46.7946 mL | |
| 5 mM | 0.9359 mL | 4.6795 mL | 9.3589 mL | |
| 10 mM | 0.4679 mL | 2.3397 mL | 4.6795 mL |