4E1RCat is a potent and dual inhibitor of eIF4E:eIF4G and eIF4E:4E-BP1 protein-protein interaction, and also inhibits the binding of eIF4G to eIF4E with IC50 of 3.2 μM. Cap-dependent translation is inhibited and eIF4F complex assembly is prevented by 4E1RCat. It was confirmed that 4E1RCat specifically inhibited 5′-cap-mediated translation by significantly inhibiting 5′-cap-mediated mCherry synthesis while having minimal effect on IRES-mediated DIAPH1-HA synthesis.
Physicochemical Properties
| Molecular Formula | C28H18N2O6 | |
| Molecular Weight | 478.45 | |
| Exact Mass | 478.116 | |
| Elemental Analysis | C, 70.29; H, 3.79; N, 5.86; O, 20.06 | |
| CAS # | 328998-25-0 | |
| Related CAS # |
|
|
| PubChem CID | 16195554 | |
| Appearance | Brown to purplish red solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 764.8±60.0 °C at 760 mmHg | |
| Flash Point | 416.3±32.9 °C | |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C | |
| Index of Refraction | 1.712 | |
| LogP | 6.28 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 6 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 36 | |
| Complexity | 902 | |
| Defined Atom Stereocenter Count | 0 | |
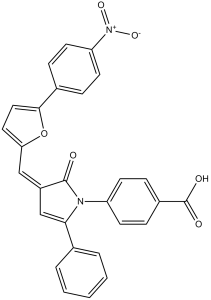
| SMILES | O=C(O)C1=CC=C(N2C(/C(C=C2C3=CC=CC=C3)=C\C4=CC=C(C5=CC=C([N+]([O-])=O)C=C5)O4)=O)C=C1 |
|
| InChi Key | BBQRBOIMSKMFFO-LTGZKZEYSA-N | |
| InChi Code | InChI=1S/C28H18N2O6/c31-27-21(16-24-14-15-26(36-24)19-6-12-23(13-7-19)30(34)35)17-25(18-4-2-1-3-5-18)29(27)22-10-8-20(9-11-22)28(32)33/h1-17H,(H,32,33)/b21-16+ | |
| Chemical Name | 4-[(3E)-3-[[5-(4-nitrophenyl)furan-2-yl]methylidene]-2-oxo-5-phenylpyrrol-1-yl]benzoic acid | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | eIF4 |
| ln Vitro | 4E1RCat is an inhibitor of eIF4E:eIF4GI interaction, with an IC50 an of ~4 μM. Additionally, 4E-BP and eIF4G binding is impeded by 4E1RCat binding to eIF4E? In a manner dependent on the cap, 4E1RCat prevents ribosome recruitment to mRNA[1]. 4E1RCat inhibits the translation of capped mRNA, while CDK1/CYCB1 initiates the translation. HeLa and U2OS cells exhibit nearly complete cap-dependent and -sensitive new protein synthesis during both mitosis and interphase, in response to 4E1RCat treatment[2]. |
| ln Vivo | 4E1RCat (15 mg/kg, i.p.) affects the chemosensitivity of Pten+/-Eμ-Myc tumors in mice. In mice, 4E1RCat (15 mg/kg, i.p.) targets translation and sensitizes Pten+/-Eμ-Myc and Tsc2+/-Eμ-Myc lymphomas to the cytotoxic effects of doxorubicin (Dxr)[1]. |
| Cell Assay | TSC2sup>+/-Eμ-Myc and Eμ-Myc lymphomas are cultured at 10sup>6 cells/mL in 96-well plates with varying concentrations of 4E1RCat (78.13 nM to 10 000 nM) and doxorubicin (Dxr) at a constant ratio of either 20:1 or 40:1. Dxr ranges from 3.9 nM to 250 nM. 24 hours later, an MTS assay is carried out. In order to achieve this, the plates are loaded with the Cell Proliferation Assay, and they are then incubated for a maximum of three hours before the OD490 is measured. The values acquired are normalized in comparison to DMSO controls [1]. |
| Animal Protocol | Mice: Female C57BL/6 mice aged 6-8 weeks are given an injection of one million secondary Pten+/-Eμ-Myc, Tsc2+/-Eμ-Myc, or Eμ-Myc lymphoma cells into their tail vein. Mice with palpable tumors are given either doxorubicin (once at 10 mg/kg) or 4E1RCat (15 mg/kg daily for 5 d) as treatment. The compounds are injected intraperitoneally (i.p.) at a ratio of 5.2% PEG 400 to 5.2% Tween 80. Doxorubicin is given once on day two of combination studies, during which either rapamycin or 4E1RCat are injected intraperitoneally (i.p.) every day for five days in a row. Every day, workers palpate animals to check for the development of tumors. The amount of time that passes before a tumor recurs is known as tumor-free survival. The log-rank test, which is presented in Kaplan-Meier format, is used to analyze data for statistical significance[1]. |
| References |
[1]. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):1046-51. [2]. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc Natl Acad Sci U S A. 2015 May 12;112(19):5875-82. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0901 mL | 10.4504 mL | 20.9008 mL | |
| 5 mM | 0.4180 mL | 2.0901 mL | 4.1802 mL | |
| 10 mM | 0.2090 mL | 1.0450 mL | 2.0901 mL |