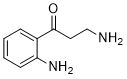
Physicochemical Properties
| Molecular Formula | C9H12N2O |
| Molecular Weight | 164.21 |
| Exact Mass | 145.052 |
| CAS # | 611-36-9 |
| Related CAS # | 611-36-9; |
| PubChem CID | 69141 |
| Appearance | White to light brown solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 313.0±15.0 °C at 760 mmHg |
| Melting Point | 200-202 °C(lit.) |
| Flash Point | 143.1±20.4 °C |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.691 |
| LogP | 2.45 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 11 |
| Complexity | 198 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | PMZDQRJGMBOQBF-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C9H7NO/c11-9-5-6-10-8-4-2-1-3-7(8)9/h1-6H,(H,10,11) |
| Chemical Name | 4-Hydroxyquinoline |
| Synonyms | 4-Hydroxyquinoline 4 Hydroxyquinoline 4Hydroxyquinoline Kynurine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | 4-Quinolone (Kynurine) promotes tumor cell survival and motility via autocrinely/paracrinely inhibiting anti-tumor immune responses via AhR. This mechanism is especially active in human brain tumors, where kynurenine production and TDO expression are both increased by AhR activation. TDO protein levels are extremely low in healthy human brains, but they rise with the aggressiveness of human brain tumors [3]. |
| References |
[1]. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents. Focus on kynurenine-3-hydroxylase. Adv Exp Med Biol. 2003;527:621-8. [2]. Cytochrome P450 Gene Regulation: Reporter Assays to Assess Aryl Hydrocarbon Receptor (HLHE76, AhR) Activation and Antagonism. Cytochrome P450. pp 157-174. [3]. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011 Oct 5;478(7368):197-203. |
| Additional Infomation |
4-quinolone is a quinolone that is 1,4-dihydroquinoline substituted by an oxo group at position 4. It is a tautomer of a quinolin-4-ol. 4-Hydroxyquinoline has been reported in Glycosmis parviflora and Glycosmis citrifolia with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~100 mg/mL (~688.90 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (17.22 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (17.22 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (17.22 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.0898 mL | 30.4488 mL | 60.8976 mL | |
| 5 mM | 1.2180 mL | 6.0898 mL | 12.1795 mL | |
| 10 mM | 0.6090 mL | 3.0449 mL | 6.0898 mL |