Physicochemical Properties
| Molecular Formula | C26H38O5 |
| Molecular Weight | 430.58 |
| Exact Mass | 386.209 |
| CAS # | 135646-98-9 |
| PubChem CID | 9867370 |
| Appearance | Typically exists as solid at room temperature |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 594.2±50.0 °C at 760 mmHg |
| Flash Point | 327.2±26.6 °C |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.608 |
| LogP | 1.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 14 |
| Heavy Atom Count | 31 |
| Complexity | 564 |
| Defined Atom Stereocenter Count | 4 |
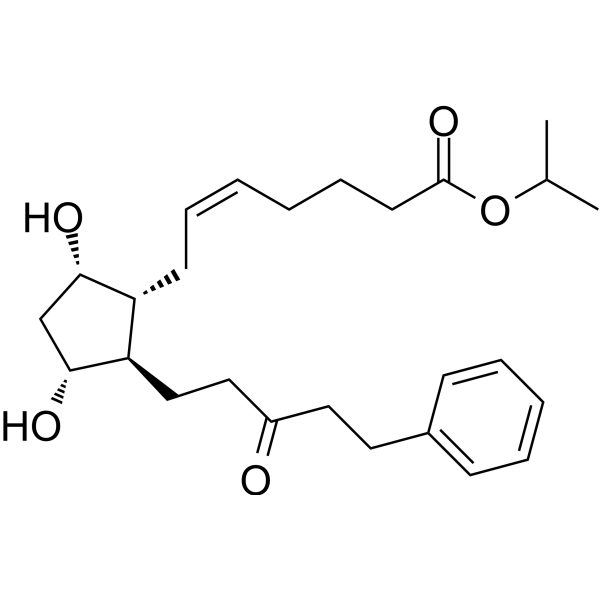
| SMILES | CC(C)OC(=O)CCC/C=C\C[C@@H]1[C@@H](CCC(=O)CCC2=CC=CC=C2)[C@@H](C[C@@H]1O)O |
| InChi Key | DKYCMQSMHPIBBZ-VIZYZFHWSA-N |
| InChi Code | InChI=1S/C26H38O5/c1-19(2)31-26(30)13-9-4-3-8-12-22-23(25(29)18-24(22)28)17-16-21(27)15-14-20-10-6-5-7-11-20/h3,5-8,10-11,19,22-25,28-29H,4,9,12-18H2,1-2H3/b8-3-/t22-,23-,24+,25-/m1/s1 |
| Chemical Name | propan-2-yl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-(3-oxo-5-phenylpentyl)cyclopentyl]hept-5-enoate |
| Synonyms | 15-Ketolatanoprost; propan-2-yl (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-(3-oxo-5-phenylpentyl)cyclopentyl]hept-5-enoate; SCHEMBL14520774; DTXSID70432091; DKYCMQSMHPIBBZ-VIZYZFHWSA-N; AKOS027326713; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | FP Receptor |
| ln Vivo | The acid of latanoprost in cynomolgus monkeys had a brief half-life in plasma and was partially converted to the latanoprost 15-keto acid. The main metabolic process was the β-oxidation of latanoprost acid[1]. |
| Animal Protocol | Latanoprost (13,14-dihydro-15(R)-17-phenyl-18,19,20-trinor-PGF2a isopropyl ester, CAS 130209-82-4 PhXA41, Xalatan) is an antiglaucoma prodrug which enhances the bioavailability of the drug into the eye compared to the corresponding acid. The pharmacokinetics and metabolism of this drug was studied in the cynomolgus monkey after daily topical administration on the eye of [13,14-(3) H] labelled latanoprost (6 micrograms per eye) during 21 days. Plasma, urine and homogenised faeces samples were purified by separation on a Sep-Pak C18 cartridge before analysis by reversed phase liquid chromatography (RP-HPLC) with one-line radioactivity detection. The maximum plasma concentration of radioactivity obtained within 10 min post dose was as a mean 7.87 +/- 3.18 ng eq./ml on day 1 and 9.31 +/- 4.21 ng eq./ml on day 21. The plasma concentration of radioactivity declined rapidly up to 3 h post-dose both on day 1 and day 21, but a small amount of tritiated water accumulated with time. The majority of the radioactivity was recovered in urine but substantial amounts were also eliminated in the faces. No latanoprost was found in plasma after repeated topical administration on the eye. The plasma profiles from HPLC separation of samples showed a rapid and complete hydrolysis of the ester. The elimination half-life of the acid of latanoprost was estimated to be 13.8 +/- 1.7 min for day 1 and 12.4 +/- 4.8 min for day 21. No induction or inhibition of the metabolism occurred after the repeated administration. By comparison with reference substances the 15-keto acid of latanoprost was found to be present in plasma and the major metabolites in urine and faeces collected during day 2 and day 20 were identified as 1,2-dinor acid of latanoprost, 1,2,3,4-tetranor acid and 1,2,3,4-tetranor lactone of latanoprost. Tritiated water was excreted in the urine and a small amount of the acid of latanoprost was excreted in the faeces. In conclusion, latanoprost was rapidly absorbed and hydrolysed to the corresponding acid after repeated topical administration to the monkey eye. The acid of latanoprost had a short half-life in plasma and it was partly converted to the 15-keto acid of latanoprost. beta-Oxidation of the acid of latanoprost was the major metabolic pathway. No induction or inhibition of the metabolism occurred upon repeated administration and no indications of accumulation of the drug or drug metabolites were observed. The pharmacokinetics of latanoprost was similar after a single and repeated topical administration. |
| References |
[1]. Pharmacokinetics of latanoprost in the cynomolgus monkey. 2nd communication: repeated topical administration on the eye. Arzneimittelforschung. 1999 Mar;49(3):234-9. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3224 mL | 11.6122 mL | 23.2245 mL | |
| 5 mM | 0.4645 mL | 2.3224 mL | 4.6449 mL | |
| 10 mM | 0.2322 mL | 1.1612 mL | 2.3224 mL |