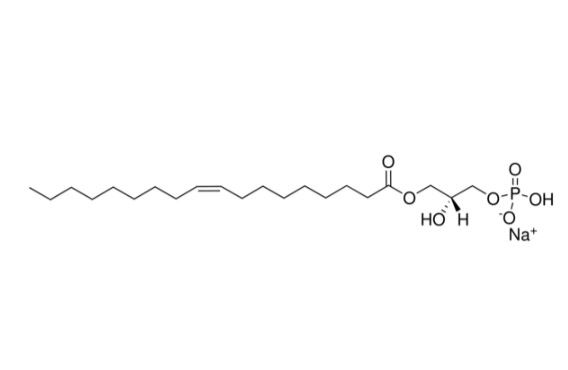
Lysophosphatidic acid is an endogenous agonist of the lysophospholipid receptors LPA1 and LPA2, a glycerophospholipid signaling ligand molecule and ligand activator for EDG-2, EDG-4, and EDG-7. It inhibits differentiation of neural stem cells (NSCs) into neurons
Physicochemical Properties
| Molecular Formula | C21H40O7P.NA |
| Molecular Weight | 458.50 |
| Exact Mass | 458.24 |
| CAS # | 325465-93-8 |
| Related CAS # | 1-Oleoyl lysophosphatidic acid; 65528-98-5 |
| PubChem CID | 44159357 |
| Appearance | White to off-white solid |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 21 |
| Heavy Atom Count | 30 |
| Complexity | 474 |
| Defined Atom Stereocenter Count | 1 |
| SMILES | CCCCCCCC/C=C\CCCCCCCC(=O)OC[C@H](COP(=O)(O)[O-])O.[Na+] |
| InChi Key | XGRLSUFHELJJAB-JGSYTFBMSA-M |
| InChi Code | InChI=1S/C21H41O7P.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(23)27-18-20(22)19-28-29(24,25)26;/h9-10,20,22H,2-8,11-19H2,1H3,(H2,24,25,26);/q;+1/p-1/b10-9-;/t20-;/m1./s1 |
| Chemical Name | sodium;[(2R)-2-hydroxy-3-[(Z)-octadec-9-enoyl]oxypropyl] hydrogen phosphate |
| Synonyms | Lysophosphatidic acid; 325465-93-8; 1-Oleoyl lysophosphatidic acid sodium salt; Sodium (R,Z)-2-hydroxy-3-(oleoyloxy)propyl hydrogenphosphate; sodium;[(2R)-2-hydroxy-3-[(Z)-octadec-9-enoyl]oxypropyl] hydrogen phosphate; MFCD00133427; 1-Oleoyl lysophosphatidic acid (sodium); 22556-62-3; 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate (sodium salt); |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | LPA receptor |
| ln Vitro |
Sodium 1-oleoyl lysophosphatidic acid (0.1-10 μM) can induce and increase the [Ca2+]i potential in rabbit osteoclasts [2]. Sodium 1-oleoyl lysophosphatidic acid (5 μM) induces the contraction of cytoclastic lamellipodia [2].
Lysophosphatidic acid (LPA) is a naturally occurring phospholipid with growth-factor-like activities [van Corven, Groenink, Jalink, Eichholtz & Moolenaar (1989) Cell 45, 45-54]. We have examined various structural analogues of LPA for their ability to stimulate DNA synthesis in quiescent fibroblasts. When the acyl-chain length is varied, the rank order of mitogenic potency is: 1-oleoyl LPA congruent to 1-palmitoyl LPA greater than 1-myristoyl LPA greater than 1-lauroyl LPA greater than 1-decanoyl LPA; the last compound shows almost no activity over the concentration range tested (1-100 microM). An ether-linked LPA (1-O-hexadecylglycerol 3-phosphate) has much decreased mitogenic activity as compared with the ester-linked analogue at concentrations less than 25 microM, and becomes cytotoxic at higher concentrations. Hexadecylphosphate, which lacks a glycerol backbone, has negligible activity. On a molar basis, diacyl phosphatidic acid (PA) is about equally potent as the corresponding LPA analogue, showing similar acyl-chain-length dependence; the data argue against the possibility that the mitogenic action of PA is due to contaminating traces of LPA. Although the short-chain analogues of LPA and PA fail to antagonize the action of long-chain (L)PAs, the polyanionic drug suramin inhibits LPA- and PA-induced, DNA synthesis in a reversible and dose-dependent manner, at concentrations [IC50 (concn. giving 50% inhibition) approximately 70 microM] that do not affect epidermal-growth-factor-induced DNA synthesis. Suramin appears to act in the early G0/G1 phase of the cell cycle, blocking immediate responses to LPA such as phosphoinositide hydrolysis. We conclude that both LPA and PA can function as growth-promoting phospholipids, with the fatty acid chain length being a major determinant of mitogenic potency. [1] Lysophosphatidic acid (LPA) is a bioactive phospholipid whose functions are mediated by multiple G protein-coupled receptors. We have shown that osteoblasts produce LPA, raising the possibility that it mediates intercellular signaling among osteoblasts and osteoclasts. Here we investigated the expression, signaling and function of LPA receptors in osteoclasts. Focal application of LPA elicited transient increases in cytosolic calcium concentration ([Ca(2+)](i)), with 50% of osteoclasts responding at approximately 400 nm LPA. LPA-induced elevation of [Ca(2+)](i) was blocked by pertussis toxin or the LPA(1/3) receptor antagonist VPC-32183. LPA caused sustained retraction of osteoclast lamellipodia and disrupted peripheral actin belts. Retraction was insensitive to VPC-32183 or pertussis toxin, indicating involvement of a distinct signaling pathway. In this regard, inhibition of Rho-associated kinase stimulated respreading after LPA-induced retraction. Real-time reverse transcription-PCR revealed transcripts encoding LPA(1) and to a lesser extent LPA(2), LPA(4), and LPA(5) receptor subtypes. LPA induced nuclear translocation of NFATc1 and enhanced osteoclast survival, effects that were blocked by VPC-32183 or by a specific peptide inhibitor of NFAT activation. LPA slightly reduced the resorptive activity of osteoclasts in vitro. Thus, LPA binds to at least two receptor subtypes on osteoclasts: LPA(1), which couples through G(i/o) to elevate [Ca(2+)](i), activate NFATc1, and promote survival, and a second receptor that likely couples through G(12/13) and Rho to evoke and maintain retraction through reorganization of the actin cytoskeleton. These findings reveal a signaling axis in bone through which LPA, produced by osteoblasts, acts on multiple receptor subtypes to induce pleiotropic effects on osteoclast activity and function [2]. |
| ln Vivo | The role of lysophosphatidic acid (LPA) in the control of emotional behavior remains to be determined. We analyzed the effects of the central administration of 1-oleoyl-LPA (LPA 18∶1) in rats tested for food consumption and anxiety-like and depression-like behaviors. For this purpose, the elevated plus-maze, open field, Y maze, forced swimming and food intake tests were performed. In addition, c-Fos expression in the dorsal periaqueductal gray matter (DPAG) was also determined. The results revealed that the administration of LPA 18∶1 reduced the time in the open arms of the elevated plus-maze and induced hypolocomotion in the open field, suggesting an anxiogenic-like phenotype. Interestingly, these effects were present following LPA 18∶1 infusion under conditions of novelty but not under habituation conditions. In the forced swimming test, the administration of LPA 18∶1 dose-dependently increased depression-like behavior, as evaluated according to immobility time. LPA treatment induced no effects on feeding. However, the immunohistochemical analysis revealed that LPA 18∶1 increased c-Fos expression in the DPAG. The abundant expression of the LPA1 receptor, one of the main targets for LPA 18∶1, was detected in this brain area, which participates in the control of emotional behavior, using immunocytochemistry. These findings indicate that LPA is a relevant transmitter potentially involved in normal and pathological emotional responses, including anxiety and depression [3]. |
| Enzyme Assay | Real-time RT-PCR Analyses Total RNA was isolated from purified bone marrow-derived osteoclasts using TRIZOL reagent and the RNeasy Mini kit (Qiagen). Primers and probes for murine LPA1 (Edg2, Mm00439145_m1), LPA2 (Edg4, Mm00469562_m1), LPA3 (Edg7, Mm00469694_m1), LPA4 (GPR23, Mm01228533_m1), LPA5 (GPR92, Mm02621109_s1), calcitonin receptor (Calcr, Mm0043227_m1), glyceraldehyde-3-phosphate dehydrogenase (Gapdh, product no. 4308313), and 18 S ribosomal RNA (product no. 4308329) were from Applied Biosystems (Gene Expression Assay). Real-time RT-PCR was performed using TaqMan One-step RT-PCR Master Mix Reagents kit (Applied Biosystems) and the ABI Prism 7900HT Sequence Detector (Applied Biosystems) according to the manufacturer's recommendations. Samples were amplified in triplicate. Dilutions of total RNA obtained from murine bone marrow-derived osteoclasts, small intestine, ovaries, and MC3T3-E1 cells were used to validate relative amplification efficiencies of primer/probe sets. The amounts of mRNA were normalized to levels of 18 S ribosomal RNA in the same samples [2]. |
| Cell Assay |
Bone Marrow-derived Osteoclasts [2] Bone marrow cells from the femurs and tibias of 6–10-week-old male C57Bl/6 mice were used to prepare osteoclasts as described previously. After isolation, cells were suspended in α-minimum essential medium supplemented with FBS (10%) and antibiotics (1%) and cultured in T75 tissue culture flasks (15 × 106 cells per flask) with recombinant human macrophage colony-stimulating factor (25 ng/ml). After 24 h, non-adherent cells were removed and resuspended in α-minimum essential medium containing FBS (10%), antibiotics (1%), macrophage colony-stimulating factor (50 ng/ml), and recombinant human RANKL (huRANKL-LZ, 100 ng/ml) and plated at 10 × 104 cells/cm2 in suspension culture dishes. The resulting cells were cultured for an additional 3 days. Cells were then suspended by incubation for 10 min in Ca2+/Mg2+-free PBS at 4 °C, and osteoclasts were enriched by unit gravity velocity sedimentation through FBS (2 times). RAW-264.7-derived Osteoclast-like Cells[2] The murine leukemic monocyte macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium containing FBS (10%) and antibiotic solution (1%). RAW 264.7 cells were cultured at a density of 1.3 × 104 cells/cm2 and treated with huRANKL-LZ (100 ng/ml) for 4 days to give rise to multinucleated osteoclast-like cells. |
| Animal Protocol |
Given the abundant distribution of the LPA receptors throughout the organism, the i.c.v. administration protocol was used to study the specific role of LPA in the brain, thereby avoiding confounding results derived from potential peripheral effects.[3] For i.c.v. injections and administration, we used a previously described protocol. Stainless steel guide cannulae aimed at the left or right lateral ventricle were implanted in the rats. The animals were anesthetized with equithesin and placed in a stereotaxic apparatus with the incisor bar set at 5 mm above the interaural line. A guide cannula (7 mm and 23-gauge) was secured to the skull using two stainless steel screws and dental cement, and the opening was closed using 30-gauge obturators. The implantation coordinates were 0.6 mm posterior to bregma, ±2.0 mm lateral, and 3.2 mm below the surface of the skull, according to the rat brain stereotaxic coordinates of Paxinos and Watson. These coordinates placed the cannula 1 mm above the ventricle. After a 7-day postsurgical recovery period, the cannula patency was confirmed through the gravity flow of isotonic saline through an 8-mm long and 30-gauge injector inserted within the guide to 1 mm beyond the tip. This procedure was used to familiarize the animals with the injection technique (sham injection). The obturator was removed from the guide cannula, and an 8 mm injector (30 gauge stainless steel tubing), connected to 70 cm of calibrated polyethylene-10 tubing, was lowered into the ventricle. The tubing was subsequently raised until the flow was initiated, and 5 µL of drug or vehicle solution was infused over a 30–60 s period. The injector remained in the guide cannula for an additional 30 s to facilitate the diffusion of the solution and subsequently was removed. The xstylet was immediately replaced. |
| References |
[1]. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281 ( Pt 1)(Pt 1):163-9. [2]. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J Biol Chem. 2010 Aug 13;285(33):25792-801. [3]. 1-Oleoyl lysophosphatidic acid: a new mediator of emotional behavior in rats. PLoS One. 2014 Jan 7;9(1):e85348. |
| Additional Infomation |
Lysophosphatidic acid (LPA) is a naturally occurring phospholipid with growth-factor-like activities [van Corven, Groenink, Jalink, Eichholtz & Moolenaar (1989) Cell 45, 45-54]. We have examined various structural analogues of LPA for their ability to stimulate DNA synthesis in quiescent fibroblasts. When the acyl-chain length is varied, the rank order of mitogenic potency is: 1-oleoyl LPA congruent to 1-palmitoyl LPA greater than 1-myristoyl LPA greater than 1-lauroyl LPA greater than 1-decanoyl LPA; the last compound shows almost no activity over the concentration range tested (1-100 microM). An ether-linked LPA (1-O-hexadecylglycerol 3-phosphate) has much decreased mitogenic activity as compared with the ester-linked analogue at concentrations less than 25 microM, and becomes cytotoxic at higher concentrations. Hexadecylphosphate, which lacks a glycerol backbone, has negligible activity. On a molar basis, diacyl phosphatidic acid (PA) is about equally potent as the corresponding LPA analogue, showing similar acyl-chain-length dependence; the data argue against the possibility that the mitogenic action of PA is due to contaminating traces of LPA. Although the short-chain analogues of LPA and PA fail to antagonize the action of long-chain (L)PAs, the polyanionic drug suramin inhibits LPA- and PA-induced, DNA synthesis in a reversible and dose-dependent manner, at concentrations [IC50 (concn. giving 50% inhibition) approximately 70 microM] that do not affect epidermal-growth-factor-induced DNA synthesis. Suramin appears to act in the early G0/G1 phase of the cell cycle, blocking immediate responses to LPA such as phosphoinositide hydrolysis. We conclude that both LPA and PA can function as growth-promoting phospholipids, with the fatty acid chain length being a major determinant of mitogenic potency. [3]
LPA is as a potent mitogen and motility factor that has been implicated in the metastasis of breast and ovarian tumors to bone. Moreover, breast cancer cells overexpressing LPA1 promote the recruitment of osteoclasts to metastatic sites and stimulate bone resorption. Our previous findings demonstrate that osteoblasts can produce LPA. This LPA may attract and activate tumor cells, as well as regulate osteoclast motility and survival. Thus, LPA released from osteoblasts may be an important autocrine and paracrine mediator, physiologically regulating skeletal development and remodeling, and pathologically contributing to metastatic bone disease.[2] |
Solubility Data
| Solubility (In Vitro) |
H2O : ~100 mg/mL (~218.10 mM) DMSO : ~1.85 mg/mL (~4.03 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1810 mL | 10.9051 mL | 21.8103 mL | |
| 5 mM | 0.4362 mL | 2.1810 mL | 4.3621 mL | |
| 10 mM | 0.2181 mL | 1.0905 mL | 2.1810 mL |