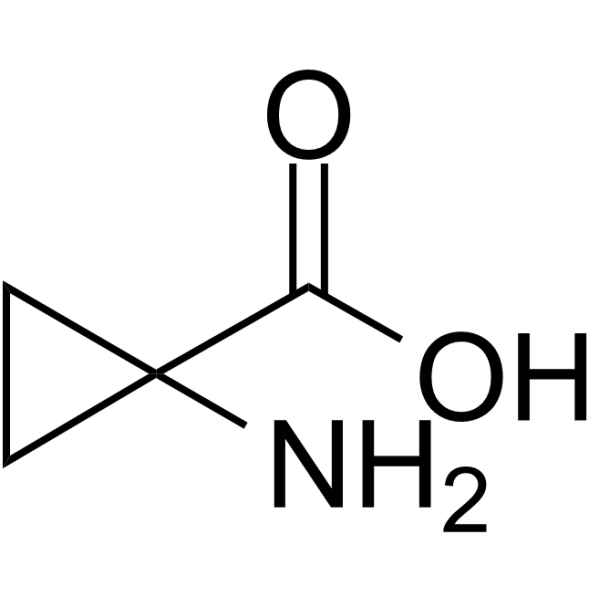
Physicochemical Properties
| Molecular Formula | C4H7NO2 |
| Molecular Weight | 101.10 |
| Exact Mass | 101.047 |
| CAS # | 22059-21-8 |
| Related CAS # | 1-Aminocyclopropane-1-carboxylic acid-d4;84392-07-4 |
| PubChem CID | 535 |
| Appearance | White to off-white solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 228.9±23.0 °C at 760 mmHg |
| Melting Point | 229-231 °C(lit.) |
| Flash Point | 92.2±22.6 °C |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.568 |
| LogP | -1.18 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Heavy Atom Count | 7 |
| Complexity | 106 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | O([H])C(C1(C([H])([H])C1([H])[H])N([H])[H])=O |
| InChi Key | PAJPWUMXBYXFCZ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C4H7NO2/c5-4(1-2-4)3(6)7/h1-2,5H2,(H,6,7) |
| Chemical Name | 1-aminocyclopropane-1-carboxylic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | NMDA receptor |
| ln Vitro | 1-Aminocyclopropanecarboxylic acid (ACPC) has been shown to protect against neuronal cell death after ischemic insult in vivo. Such results can be correlated with in vitro assays in which ACPC protected neurons against glutamate-induced neurotoxicity by reducing the activity of N-methyl-D-aspartate (NMDA) channel activation. Electrophysiological studies have determined that ACPC inhibits NMDA receptor activity by acting as a glycine-binding site partial agonist. In this study, rapid drug perfusion combined with whole-cell voltage-clamp was used to elicit and measure the effects of ACPC on NMDA receptor-mediated responses from cultured hippocampal neurons and cerebellar granule cells. The ACPC steady-state dose-response curve had both stimulatory and inhibitory phases. Half-maximal activation by ACPC as a glycine-site agonist was 0.7 to 0.9 microM. Half-maximal inhibition by ACPC was dependent on NMDA concentration. Peak responses to a >100 microM ACPC pulse in the presence of 1 microM glutamate were similar to those of glycine but decayed to a steady-state amplitude below that of glycine. The removal of ACPC initially caused an increase in inward current followed by a subsequent decrease to baseline levels. This suggests that relief of low-affinity antagonism occurs before high-affinity agonist dissociation. Simulations of ACPC action by a two glutamate-binding site/two glycine-binding site model for NMDA channel activation in conjunction with the concurrent role of ACPC as a glycine-site full agonist and glutamate-site competitive antagonist were able to successfully approximate experimental results. [1] |
| ln Vivo | 1-Aminocyclopropanecarboxylic acid (ACPC) has been shown to protect neurons against glutamate-induced neurotoxicity by reducing N-methyl-D-aspartate (NMDA) receptor activation. Recent studies have demonstrated that several antagonists of NMDA receptors have important cardiovascular effects. In this study, we examined whether the cardiovascular effects of ACPC involve the role of heme oxygenase-1 (HO-1) and its antioxidant effect in stroke-prone spontaneously hypertensive rats (SHRSP). Male SHRSP were divided into two groups: a control group and an ACPC group administered ACPC at 50 mg/kg per day for 4 weeks by peritoneal injection. Systolic blood pressure (SBP) and mortality of stroke were significantly lower in the ACPC group than in the control group. Urinary Na(+) and Cl(-) excretion and plasma superoxide dismutase (SOD) activity were increased in the ACPC group. Western analysis detected proteins that were immunoreactive to anti-nitrotyrosine antibody and showed lower levels of expression in the cerebral cortex compared to that in the control group. Immunohistochemical analysis revealed that 8-hydroxy-2'-deoxyguanosine (8-OHdG) formation in the hippocampus and cerebral cortex was reduced in the ACPC group. Quantitative reverse-transcription-polymerase chain reaction (RT-PCR) showed that administration of ACPC also significantly decreased the expression of neuronal nitric oxide synthase (nNOS) mRNA in the hippocampus and endotherial nitric oxide synthase (eNOS) mRNA in the cerebral cortex, and drastically increased HO-1 mRNA in the cerebral cortex. Enhanced HO-1 staining on sections from the hippocampus and cerebral cortex was observed in the ACPC group. These data suggest that the normalization by ACPC of blood pressure elevation and mortality of stroke involves induction of the expression of HO-1, which exerts antioxidant and vascular relaxation effects, in SHRSP [3]. |
| Animal Protocol |
Study 1 [3] Male SHRSP/Izm at 6 weeks of age were divided into two groups (each n=10): a control group (administered distilled water by peritoneal injection) and an ACPC group (administered ACPC at 50 mg/kg per day for 4 weeks by peritoneal injection; ACPC was used. All groups consumed a nonpurified laboratory diet (Funabashi Farm, Chiba, Japan). All rats were housed 2 per cage. Body weight and blood pressure were measured before allocation to groups to ensure homogeneity of weight and blood pressure. The environment was controlled at 22±1°C and with a 12-h light-dark cycle. Study 2 [3] Male SHRSP/Izm at 6 weeks of age were divided into two groups (each n=10): a control group and an ACPC group. All rats were housed as in Study 1. The survival rate of the rats was determined. |
| Toxicity/Toxicokinetics | rat LD intraperitoneal >500 mg/kg Biochemical Pharmacology., 5(108), 1960 [PMID:13880910] |
| References |
[1]. Putative partial agonist 1-aminocyclopropanecarboxylic acid acts concurrently as a glycine-site agonist and a glutamate-site antagonist at N-methyl-D-aspartate receptors. Mol Pharmacol. 1999 Dec;56(6):1207-18. [2]. Bleecker AB and Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1-18. [3]. 1-Aminocyclopropanecarboxylic Acid, an Antagonist of N-Methyl-D-Aspartate Receptors, Causes Hypotensive and Antioxidant Effects with Upregulation of Heme Oxygenase-1 in Stroke-Prone Spontaneously Hypertensive Rats. Hypertens Res, 2007,30, 249–257. |
| Additional Infomation |
1-aminocyclopropanecarboxylic acid/ACPC is a non-proteinogenic alpha-amino acid consisting of cyclopropane having amino and carboxy substituents both at the 1-position. It has a role as a plant metabolite and a member of ethylene releasers. It is a monocarboxylic acid and a non-proteinogenic alpha-amino acid. It is functionally related to a cyclopropanecarboxylic acid. It is a conjugate acid of a 1-aminocyclopropanecarboxylate. It is a tautomer of a 1-aminocyclopropanecarboxylic acid zwitterion. 1-Aminocyclopropane-1-carboxylate has been reported in Glycine max, Streptomyces, and other organisms with data available. In conclusion, our findings suggest that the administration of ACPC attenuated the development of hypertension and stroke through antioxidant effects in SHRSP, because we observed decreased protein nitrotyrosine, which is related to nNOS and eNOS expression and NO bioavailability, and an increase in plasma SOD activity. Furthermore, the increase of HO-1 expression induced by ACPC not only enhances COdependent relaxation of the cerebral arteries and improves the regulation of blood pressure, but also generates bilirubin, which exerts antioxidant cytoprotective effects. [3] |
Solubility Data
| Solubility (In Vitro) |
H2O: 25 mg/mL (247.28 mM) DMSO: < 1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 100 mg/mL (989.12 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 9.8912 mL | 49.4560 mL | 98.9120 mL | |
| 5 mM | 1.9782 mL | 9.8912 mL | 19.7824 mL | |
| 10 mM | 0.9891 mL | 4.9456 mL | 9.8912 mL |