Eravacycline (formerly known as TP-434; TP434; trade name: Xerava) is a novel fluorocycline with a broad-spectrum of antimicrobial activity against panels of recently isolated aerobic and anaerobic Gram-negative and Gram-positive bacteria. It was approved by FDA in August 2018 to treat complicated intra-abdominal infections in patients 18 years of age and older. Eravacycline showed potent broad-spectrum activity against 90% of the isolates (MIC90) in each panel at concentrations ranging from ≤0.008 to 2 μg/ml for all species panels except those of Pseudomonas aeruginosa and Burkholderia cenocepacia (MIC90 values of 32 μg/ml for both organisms). The antibacterial activity of eravacycline was minimally affected by expression of tetracycline-specific efflux and ribosomal protection mechanisms in clinical isolates. Furthermore, eravacycline was active against multidrug-resistant bacteria, including those expressing extended-spectrum β-lactamases and mechanisms conferring resistance to other classes of antibiotics, including carbapenem resistance. Eravacycline has the potential to be a promising new intravenous (i.v.)/oral antibiotic for the empirical treatment of complicated hospital/health care infections and moderate-to-severe community-acquired infections.
Physicochemical Properties
| Molecular Formula | C27H31FN4O8 |
| Molecular Weight | 558.56 |
| Exact Mass | 558.212 |
| Elemental Analysis | C, 58.06; H, 5.59; F, 3.40; N, 10.03; O, 22.91 |
| CAS # | 1207283-85-9 |
| Related CAS # | Eravacycline dihydrochloride;1334714-66-7 |
| PubChem CID | 54726192 |
| Appearance | Solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 868.1±65.0 °C at 760 mmHg |
| Flash Point | 478.8±34.3 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.711 |
| LogP | 0.25 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 40 |
| Complexity | 1200 |
| Defined Atom Stereocenter Count | 4 |
| SMILES | FC1C([H])=C(C(=C2C=1C([H])([H])[C@@]1([H])C(=C2O[H])C([C@@]2(C(=C(C(N([H])[H])=O)C([C@]([H])([C@@]2([H])C1([H])[H])N(C([H])([H])[H])C([H])([H])[H])=O)O[H])O[H])=O)O[H])N([H])C(C([H])([H])N1C([H])([H])C([H])([H])C([H])([H])C1([H])[H])=O |
| InChi Key | HLFSMUUOKPBTSM-ISIOAQNYSA-N |
| InChi Code | InChI=1S/C27H31FN4O8/c1-31(2)20-13-8-11-7-12-14(28)9-15(30-16(33)10-32-5-3-4-6-32)21(34)18(12)22(35)17(11)24(37)27(13,40)25(38)19(23(20)36)26(29)39/h9,11,13,20,34,36-37,40H,3-8,10H2,1-2H3,(H2,29,39)(H,30,33)/t11-,13-,20-,27-/m0/s1 |
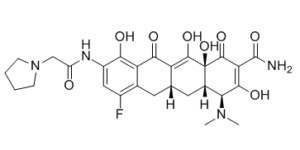
| Chemical Name | (4S,4aS,5aR,12aS)-4-(Dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-((pyrrolidin-1-ylacetyl)amino)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide |
| Synonyms | TP-434-046; TP 434-046; TP434-046; TP-434; TP 434; TP434; Eravacycline free base |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Tetracycline |
| ln Vitro | Strong antibiotic eravacycline works against isolates of A. baumannii, including those resistant to levofloxacin, imipenem/meropenem, sulbactam, and amikacin/tobramycin. Eravacycline exhibits higher activity than colistin, levofloxacin, amikacin, tobramycin, and comparators in the tetracycline class. The MIC50/90 values of eravacycline are 0.5/1 mg/L [1]. Six E. coli strains with MICs ranging from 0.125 to 0.25 mg/L exhibit inhibitory effects when exposed to eravacycline[2]. Eravacycline dihydrochloride is a synthetic antibiotic that binds to the 30S ribosomal subunit to prevent bacteria from synthesizing proteins. Eravacycline exhibits good activity against significant gram-positive pathogens, such as methicillin-resistant S. aureus, and broad spectrum activity against gram-negative bacteria in the panel, with the exception of P. aeruginosa. Additionally, eravacycline exhibits strong ribosomal inhibition[3].In all species panels, eravacycline exhibits strong broad-spectrum activity against 90% of the isolates (MIC90) at concentrations ranging from ≤0.008 to 2 μg/mL, with the exception of Pseudomonas aeruginosa and Burkholderia cenocepacia, which both have MIC90 values of 32 μg/mL. Eravacycline exhibits efficacy against bacteria that are resistant to multiple drugs, such as those that express extended-spectrum β-lactamases and mechanisms that confer resistance to other antibiotic classes, such as carbapenem resistance[4]. |
| ln Vivo | Every 12 hours, mice are given eravacycline at two-fold increasing doses (ranging from 3.125 to 50 mg/kg). The 1-log kill endpoint and net stasis are associated with mean fAUC/MIC magnitudes of 32.60±10.85 and 27.97±8.29, respectively[2]. Eravacycline effectively combats both Gram-positive and Gram-negative pathogens that are clinically significant in a variety of murine infection models. In mouse models of septicemia, eravacycline is effective; it shows 50% protective dose values of ≤1 mg/kg of body weight once daily (q.d.) against Streptococcus pyogenes and Tetracycline-resistant isolates of methicillin-resistant S. aureus (MRSA). In relation to Escherichia coli isolates, the PD50 values range from 1.2 to 4.4 mg/kg q.d[5]. |
| Animal Protocol |
Rats [3] Sprague-Dawley rats are used to determine pharmacokinetic (PK) parameters. After fasting for at least 12 hours, the animals receive a single oral dose of eravacycline (10 mg/kg) or an IV dose (1 mg/kg), and then they participate in a 24-hour sampling scheme. Using the relevant standard curves, TurboIonspray LC/MSMS analysis determines the concentrations of the dosing solution and plasma. Noncompartmental analysis is used to calculate PK parameters. |
| References |
[1]. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents. 2017 Jul 10. [2]. In Vivo Pharmacodynamic Target Assessment of Eravacycline against Escherichia coli in a Murine Thigh Infection Model. Antimicrob Agents Chemother. 2017 Jun 27;61(7). [3]. Fluorocyclines. 1. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem. 2012 Jan 26;55(2):597-605. [4]. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013 Nov;57(11):5548-58. [5]. Eravacycline (TP-434) is efficacious in animal models of infection. Antimicrob Agents Chemother. 2015 May;59(5):2567-71. |
| Additional Infomation |
Eravacycline is a member of tetracyclines. Eravacycline is a Tetracycline-class Antibacterial. See also: Eravacycline (annotation moved to); Eravacycline Dihydrochloride (annotation moved to). Drug Indication Xerava is indicated for the treatment of complicated intra-abdominal infections (cIAI) in adults. Consideration should be given to official guidance on the appropriate use of antibacterial agents. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7903 mL | 8.9516 mL | 17.9032 mL | |
| 5 mM | 0.3581 mL | 1.7903 mL | 3.5806 mL | |
| 10 mM | 0.1790 mL | 0.8952 mL | 1.7903 mL |