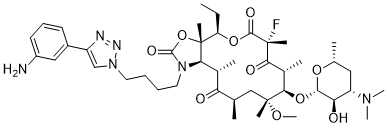
Solithromycin (CEM-101; OP1068; CEM101; OP-1068) is a novel and potent ketolide antibiotic with improved antimicrobial effectiveness. It has a broad spectrum of activity against Gram-positive respiratory tract pathogens, including macrolide-resistant strains. Solithromycin is being studied in clinical trials for the treatment of community-acquired pneumonia (CAP) and other infections.
Physicochemical Properties
| Molecular Formula | C43H65FN6O10 |
| Molecular Weight | 845.01 |
| Exact Mass | 844.474 |
| Elemental Analysis | C, 61.12; H, 7.75; F, 2.25; N, 9.95; O, 18.93 |
| CAS # | 760981-83-7 |
| PubChem CID | 25242512 |
| Appearance | Solid powder |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 969.2±75.0 °C at 760 mmHg |
| Flash Point | 539.9±37.1 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.591 |
| LogP | 3.44 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 11 |
| Heavy Atom Count | 60 |
| Complexity | 1530 |
| Defined Atom Stereocenter Count | 13 |
| SMILES | CC[C@@H]1[C@@]2([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H](C(=O)[C@](C(=O)O1)(C)F)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)OC)C)C)N(C(=O)O2)CCCCN4C=C(N=N4)C5=CC(=CC=C5)N)C |
| InChi Key | IXXFZUPTQVDPPK-QIFQIIIXSA-N |
| InChi Code | InChI=1S/C43H65FN6O10/c1-12-32-43(8)35(50(40(55)60-43)19-14-13-18-49-23-30(46-47-49)28-16-15-17-29(45)21-28)26(4)33(51)24(2)22-41(6,56-11)37(27(5)36(53)42(7,44)39(54)58-32)59-38-34(52)31(48(9)10)20-25(3)57-38/h15-17,21,23-27,31-32,34-35,37-38,52H,12-14,18-20,22,45H2,1-11H3/t24-,25-,26-,27+,31+,32-,34-,35-,37-,38+,41-,42-,43-/m1/s1 |
| Chemical Name | 3aS,4R,7R,9R,10R,11R,13R,15S,15aR)-1-(4-(4-(3-aminophenyl)-1H-1,2,3-triazol-1-yl)butyl)-10-(((2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4-ethyl-7-fluoro-11-methoxy-3a,7,9,11,13,15-hexamethyloctahydro-1H-[1]oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(7H,9H)-tetraone |
| Synonyms | CEM 101; OP1068; OP1068; OP 1068; Solithromycin;CEM101; CEM101 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Macrolide |
| ln Vitro | The half-life of solithromycin on the release of TNFα and CXCL8 is 41.6 μM and 78.2 μM, respectively. MMP9 activity is significantly reduced by solithromycin, with an IC50 of 14.9 μM[2]. In monocytic U937 and PBMC cells, levofloxacin (0-333 μM; 72 hours) inhibits the release of TNFα induced by lipopolysaccharide and the activity of matrix metalloproteinase 9 (MMP9) induced by phorbol 12-myristate 13-acetate (PMA). It has no effect on cell viability[2]. |
| ln Vivo | After exposing C57BL/6J mice to cigarette smoke for eight days, oral administration of levofloxacin (100 mg/kg) inhibits the accumulation of inflammatory cells and the production of pro-MMP9[2]. |
| Animal Protocol |
Animal Model: C57BL/6J mice (male, 4 weeks)[2] Dosage: 100 mg/kg Administration: Oral administration; every day; for 8 days Result: prevented the production of pro-MMP9 and neutrophilia brought on by cigarette smoke. |
| References |
[1]. Solithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus,Streptococcus pneumoniae, and Haemophilus influenzae. Antimicrob Agents Chemother. 2013 Apr;57(4):1632-1637. [2]. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-κB inhibition. J Pharmacol Exp Ther. 2013 Apr;345(1):76-84. |
| Additional Infomation |
Solithromycin is an aminoglycoside. Solithromycin is a ketolide antibiotic undergoing clinical development for the treatment of community-acquired pneumonia (CAP) and other infections. Drug Indication Investigated for the treatment of community-acquired pneumonia (CAP). Treatment of gonococcal infection Treatment of anthrax, Treatment of tularaemia, Treatment of bacterial pneumonia |
Solubility Data
| Solubility (In Vitro) |
DMSO : 32~100 mg/mL ( 37.87~118.34 mM )
Ethanol : ~25 mg/mL H2O : ~1 mg/mL (~1.18 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (2.96 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (2.96 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 3: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (2.96 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1834 mL | 5.9171 mL | 11.8342 mL | |
| 5 mM | 0.2367 mL | 1.1834 mL | 2.3668 mL | |
| 10 mM | 0.1183 mL | 0.5917 mL | 1.1834 mL |