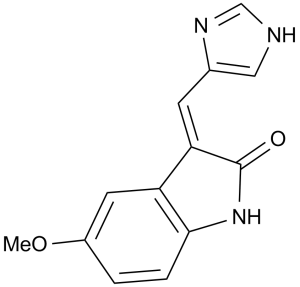
SU 9516 (SU-9516; SU9516), a 3-substituted indolinone, is a potent and selective Cyclin-dependent kinases (CDKs) inhibitor with potential antineoplastic activity. With respective IC50s of 22 nM, 40 nM, and 200 nM, it inhibits CDK2, CDK1, and CDK4. SU 9516 (5 μM) reduced pRb's phosphorylation in RKO cells by 52%, specifically in the cdk2-specific region. In SW480 cells, however, SU9516 (5 μM) significantly reduced the phosphorylation of pRb that is specific to either CDK2 or CDK4, by 64% and 49%, respectively. Moreover, G0-G1 or G2-M block and dose-dependent induction of apoptosis were the outcomes of SU 9516 (5 μM). Time-dependently, SU9516 (5 μM) prevented pRb from dissociating from E2F1 in human colon cancer cells HT-29, SW480, and RKO.
Physicochemical Properties
| Molecular Formula | C13H11N3O2 | |
| Molecular Weight | 241.25 | |
| Exact Mass | 241.085 | |
| Elemental Analysis | C, 64.72; H, 4.60; N, 17.42; O, 13.26 | |
| CAS # | 377090-84-1 | |
| Related CAS # |
|
|
| PubChem CID | 5289419 | |
| Appearance | Light brown to brown solid powder | |
| Density | 1.4±0.1 g/cm3 | |
| Boiling Point | 601.5±55.0 °C at 760 mmHg | |
| Flash Point | 317.6±31.5 °C | |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C | |
| Index of Refraction | 1.696 | |
| LogP | 1.02 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 18 | |
| Complexity | 369 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O(C([H])([H])[H])C1C([H])=C([H])C2=C(C=1[H])/C(=C(\[H])/C1=C([H])N=C([H])N1[H])/C(N2[H])=O |
|
| InChi Key | FUOLFAHJLGZFME-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C27H24N4O2/c28-23-13-7-8-14-24(23)31-27(33)25-16-15-21(18-29-25)30-26(32)22(20-11-5-2-6-12-20)17-19-9-3-1-4-10-19/h1-16,18,22H,17,28H2,(H,30,32)(H,31,33) | |
| Chemical Name | N-(2-aminophenyl)-5-(2,3-diphenylpropanoylamino)pyridine-2-carboxamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | CDK2 (IC50 = 22 nM); CDK1 (IC50 = 40 nM); CDK4 (IC50 = 200 nM); PDGFr (IC50 = 18000 nM) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | Polypropylene plates with 96 wells are used for kinase assays. A 40 μL volume containing 10 mM MgCl2, 1 mM DTT, 0.01% Triton X-100, 10% glycerol, and 2 μg of histone H1 at a final concentration of 10 μM [-33P]ATP (0.2 μCi/well) is used in each reaction. 20 μL of the enzyme (6 ng cdk2/well, or 1.6 nM at the end concentration) is added to start the reaction. The enzyme has been diluted 1:50–1:200 in the same buffer and has been left to proceed for one hour at room temperature. 25 μL of the reaction mixture is transferred to P30 phosphocellulose filter mat paper, and the reaction is stopped by adding 0.01 mL of 10% phosphoric acid. The filter mat is air dried, rinsed three times with 1.0% phosphoric acid, and then its radioactivity is measured using a liquid scintillation counter. | ||
| Cell Assay | In 96-well plates, RKO and SW480 cells are seeded at a density of 1×104 cells/well in duplicates (n = 6) and left to attach overnight. Following a 24-hour addition of SU9516 at concentrations ranging from 0.05 μM to 50.00 μM, the cells are twice washed with PBS and restocked with full media. Using a modified SRB cytotoxicity assay, the cells are fixed at 0, 4, and 7 days after the drug is removed and their protein levels are measured. After being fixed for one hour in 10% trichloroacetic acid, the cells are cleaned in distilled water and stained for thirty minutes in 0.4% SRB/acetic acid. After solubilizing in 10 mM Tris (pH 9), the cells are rinsed in 0.1% acetic acid and examined at 595 nm using a Bio-Rad 360 microplate reader. Every experiment is run through three times or more. | ||
| Animal Protocol |
|
||
| References |
[1]. A novel cdk2-selective inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 2001 Aug 15;61(16):6170-7. [2]. SU9516, a cyclin-dependent kinase 2 inhibitor, promotes accumulation of high molecular weight E2F complexes in human colon carcinoma cells. Biochem Pharmacol. 2002 Oct 1;64(7):1091-100. [3]. The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism. Mol Pharmacol. 2006 Aug;70(2):645-55. |
||
| Additional Infomation | 3-(1H-imidazol-5-ylmethylidene)-5-methoxy-1H-indol-2-one is a member of indoles. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3.25 mg/mL (13.47 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3.25 mg/mL (13.47 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 32.5 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1451 mL | 20.7254 mL | 41.4508 mL | |
| 5 mM | 0.8290 mL | 4.1451 mL | 8.2902 mL | |
| 10 mM | 0.4145 mL | 2.0725 mL | 4.1451 mL |