Physicochemical Properties
| Molecular Formula | C13H20CLN3 |
| Molecular Weight | 253.77 |
| Exact Mass | 253.135 |
| CAS # | 4749-61-5 |
| PubChem CID | 185944 |
| Appearance | White to off-white solid powder |
| Boiling Point | 325.1ºC at 760 mmHg |
| Flash Point | 150.4ºC |
| LogP | 2.821 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Heavy Atom Count | 17 |
| Complexity | 238 |
| Defined Atom Stereocenter Count | 0 |
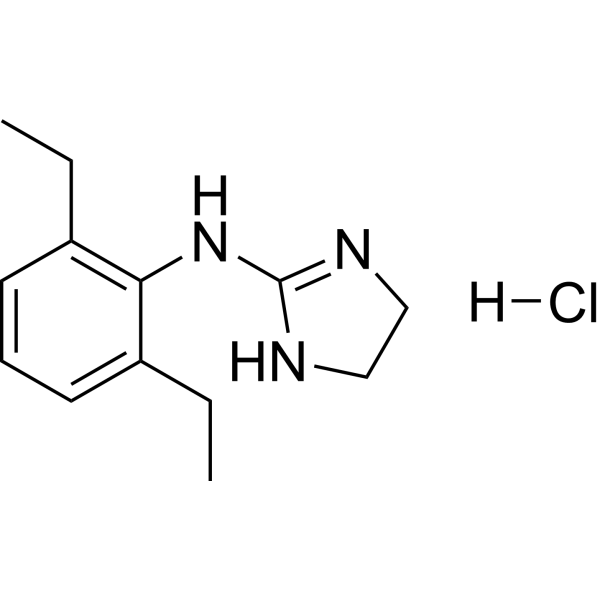
| SMILES | CCC1=C(NC2NCCN=2)C(CC)=CC=C1.Cl |
| InChi Key | ZLRWFGBEDNTMEU-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C13H19N3.ClH/c1-3-10-6-5-7-11(4-2)12(10)16-13-14-8-9-15-13;/h5-7H,3-4,8-9H2,1-2H3,(H2,14,15,16);1H |
| Chemical Name | N-(2,6-diethylphenyl)-4,5-dihydro-1H-imidazol-2-amine;hydrochloride |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | α2-adrenergic receptor |
| ln Vitro | B16F10 cell viability, proliferation, and mitochondrial function are all decreased by ST91 [2]. |
| ln Vivo | Rats treated intrathecally with ST91 exhibit antinociceptive effects [1]. |
| References |
[1]. Graham BA, et, al. Synergistic interactions between two alpha(2)-adrenoceptor agonists, dexmedetomidine and ST-91, in two substrains of Sprague-Dawley rats. Pain. 2000 Mar;85(1-2):135-43. [2]. Maccari S, et, al. α-Adrenoceptor stimulation attenuates melanoma growth in mice. Br J Pharmacol. 2022 Apr;179(7):1371-1383. [3]. Stone LS, et, al. ST91 [2-(2,6-diethylphenylamino)-2-imidazoline hydrochloride]-mediated spinal antinociception and synergy with opioids persists in the absence of functional alpha-2A- or alpha-2C-adrenergic receptors. J Pharmacol Exp Ther. 2007 Dec;323(3):899-906. |
| Additional Infomation | See also: St 91 (annotation moved to). |
Solubility Data
| Solubility (In Vitro) | DMSO: 125 mg/mL (492.57 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (8.20 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (8.20 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9406 mL | 19.7029 mL | 39.4058 mL | |
| 5 mM | 0.7881 mL | 3.9406 mL | 7.8812 mL | |
| 10 mM | 0.3941 mL | 1.9703 mL | 3.9406 mL |