Physicochemical Properties
| Molecular Formula | C10H12N6O |
| Molecular Weight | 232.24188 |
| Exact Mass | 232.107 |
| Elemental Analysis | C, 51.72; H, 5.21; N, 36.19; O, 6.89 |
| CAS # | 6975-75-3 |
| Related CAS # | 6975-75-3; 866409-68-9; |
| PubChem CID | 228619 |
| Appearance | Light yellow to yellow solid powder |
| Density | 1.5g/cm3 |
| Boiling Point | 420.4ºC at 760 mmHg |
| Flash Point | 208ºC |
| Index of Refraction | 1.715 |
| LogP | 3.16 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 17 |
| Complexity | 266 |
| Defined Atom Stereocenter Count | 0 |
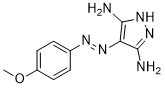
| SMILES | COC1=CC=C(C=C1)N=NC2=C(NN=C2N)N |
| InChi Key | QNRNTYHAOBVOKW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C10H12N6O/c1-17-7-4-2-6(3-5-7)13-14-8-9(11)15-16-10(8)12/h2-5H,1H3,(H5,11,12,15,16) |
| Chemical Name | 4-(4-Methoxy-phenylazo)-1H-pyrazole-3,5-diamine |
| Synonyms | NSC 21678; NSC-21678; 6975-75-3; ILK-IN-3; 4-((4-Methoxyphenyl)diazenyl)-1H-pyrazole-3,5-diamine; 866409-68-9; 4-[(4-methoxyphenyl)hydrazono]-4h-pyrazole-3,5-diamine; MLS000737992; 4-(4-Methoxy-phenylazo)-1H-pyrazole-3,5-diamine; NSC21678; ILK-IN-3; QLT-0267; QLT 0267; QLT0267; QLT-267; QLT 267; QLT267 |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Integrin linked kinase (ILK); DYRK1 |
| ln Vitro |
The activities of DYRK1, GSK3α/β, and CDK5/p25 are inhibited by QLT-0267 (ILK-IN-3)(compound 4, 10 μM) to 17%, 51%, 47%, and 95%[2].
Effects of EMO on the expression of nephrin, desmin and ILK in HG-stimulated podocytes [2] In order to investigate the effects of EMO on HG-induced EMT in podocytes, we examined the epithelial marker nephrin, the mesenchymal marker desmin and ILK. Compared with incubation in NG, exposure to HG for 24 h elevated the protein expression of both desmin and ILK while reduced the nephrin protein expression in podocytes. Podocytes treated with EMO showed a significant decrease in desmin and ILK protein expression, as well as an increase in nephrin expression. Likewise, ILK inhibitor QLT0267 also reduced desmin protein expression and elevated nephrin protein expression in HG-stimulated podocytes (Fig. 1). Immunofluorescence analysis further confirmed that HG induced an increment in desmin expression in podocytes. However, the HG-induced increase in desmin expression was alleviated by EMO or QLT0267 treatment (Fig. 2). These results showed that EMO partially restored epithelial marker nephrin expression and inhibited mesenchymal marker desmin and ILK expression in HG-stimulated podocytes. Effects of EMO on the mRNA expression of nephrin, desmin and ILK in HG-stimulated podocytes [2] HG for 24 h significantly increased the mRNA expression of both desmin and ILK while reduced nephrin mRNA expression in podocytes. However, the HG-induced decrease in nephrin mRNA expression was partially restored by EMO treatment (Fig. 3). EMO also significantly reduced desmin and ILK mRNA expression in HG-stimulated podocytes. ILK inhibitor QLT0267 had similar effects on mRNA expression of desmin and nephrin in HG-stimulated podocytes. |
| ln Vivo |
In an orthotopic LCC6 model, ILK-IN-3 (QLT0267) (200 mg/kg, interface, 28 days) inhibits tumor growth [1]. Assessing whether the metastatic model could be sensitized to docetaxel by combining its use with the ILK inhibitor QLT0267. [1] The results presented thus far indicate that the metastatic disease is least sensitive to Dt when used at a dose close to the maximum tolerated dose. This may be explained in part by limited drug delivery to tissues with established disease (Fig. 3). To determine whether treatment of the experimental metastatic model can be improved, Dt was used in combination QLT0267, an agent that targets ILK and can sensitize cells to cytotoxic agents by inhibiting the AKT pathway. Previously it was shown that QLT0267 improved the efficacy of Dt when used to treat the orthotopic LCC6 model. In the present study, QLT0267 and Dt were used alone and in combination to treat animals with established LCC6WT-luc metastatic tumors. Metastatic disease (i.c.) was established and treated as described in the Methods. The results of this in vivo efficacy study have been summarized in Figure 6. Tumor growth was monitored using BLI (Fig. 6A and B). Survival (Fig. 6C) was determined based on the time in days before animals were terminated due to poor health status. Tumors in animals treated with Dt, QLT0267 and Dt/QLT0267 showed reduced bioluminescence on day 21 post cell injection when compared to vehicle control treated mice. Quantification of total light flux demonstrated that the tumor burden on day 21 was significantly less in mice that had received treatment as compared to mice treated with the vehicle control (p < 0.0001), however animals treated with the combination of Dt/QLT0267 did not exhibit benefits that were significantly better than that achieved with Dt alone. This is likely because Dt treatment was so effective when assessing therapy at this time point. Interestingly, analysis of survival data (Fig. 6C) (as defined by animal health) suggested that a reduction in tumor burden, as exemplified by treatment with Dt, has minimal effect on survival. A possible explanation for this may be that 10 mg/kg was able to slow tumor growth so that on day 21 when images were taken minimal disease burden is observed, but later images illustrate that disease continues to develop in Dt treated animals after day 21. Furthermore, for animals treated with QLT0267 (200 mg/kg) the median survival time was 31 days for LCC6WT-luc (n = 5) as compared to 23 days for control animals (n = 13). Animals treated with Dt exhibited a median survival time of 45 days for LCC6WT-luc. Animals treated with the QLT0267/Dt combination exhibited a median survival time that was not significantly better than that achieved with QLT0267 alone and, surprisingly, suggested that the treatment outcomes when using the drug combination was worse than that achieved with Dt alone. Effects of EMO on biochemical markers in diabetic rats [3] As shown in Figure 4, blood glucose (BG) level was significantly higher in diabetic rats compared with nondiabetic control animals. However, no differences in BG level was observed between EMO treated and untreated STZ-induced diabetic rats (Fig. 4A). Moreover, the STZ-induced diabetic rats showed severe urinary albumin/creatinine ratio (ACR) when compared with the nornmal control rats (P < 0.05). However, treatment with EMO or QLT0267 significantly reduced urinary ACR (expressed as mg/g Cr ) in STZ-induced diabetic rats, which indicated that EMO reduced albuminuria similar to QLT0267. Effects of EMO on renal histopathology and podocyte foot process effacement in diabetic rats. [3] The STZ-induced diabetic rats showed focal mesangial matsrix expansion when compared with normal control rats. However, EMO treatment significantly attenuated mesangial expansion compared to the untreated STZ-induced diabetic rats (Fig. 5). Moreover, observation of podocyte ultrastructure by electron microscopy (EM) showed apparent podocyte foot process effacement in diabetic rats, whereas the rats treated with EMO revealed a marked decrease in podocyte foot process effacement. Inhibition of ILK with QLT0267 had similar effects on renal histopathology and podocyte foot process effacement to that of EMO (Fig. 5). Effects of EMO on expression of nephrin, desmin and ILK in diabetic rats [3] By immunohistochemical staining, we observed that the expression of desmin and ILK was markedly elevated while the nephrin expression was reduced in the renal tissue from STZ-induced diabetic rats when compared with the normal control rats. However, all of these abnormalities were partially restored by EMO treatment. ILK inhibitor QLT0267 had similar effects on the renal expression of nephrin, desmin and ILK in diabetic rats (Fig. 6). The results of Western blot analysis further confirmed these findings (Fig. 7). The protein expression of desmin and ILK was increased in kidneys from diabetic rats when compared with the normal control rats, which was partially abrogated by treatment with EMO. Moreover, diabetic rats revealed a significant decrease in protein level of nephrin while the rats received EMO showed a marked increase in protein production of nephrin (Fig. 7). Futhermore, changes in mRNA expression of nephrin, desmin and ILK were quantified by real-time quantitative RT-PCR. Similarly, renal cortical mRNA expression of desmin and ILK were up-regulated while the nephrin expression was down-regulated in diabetic rats, which was partially attenuated by treatment with EMO (Fig. 8). The above results demonstrated that the protective effect of EMO on podocyte EMT and subsequent podocyte depopulation in diabetic rats was associated with the inhibition of ILK and desmin as well as upregulatiotion of nephrin. |
| Cell Assay |
In-vitro studies: Cell culture and EMO treatment [3] Conditionally immortalized mouse podocytes were kindly provided by Dr. Peter Mundel and were conducted as described previously [12]. In brief, podocytes were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100U/ml penicillin and 100μg/ml streptomycin. Cells were grown at 33°C in the presence of 10 U/ml mouse recombinant interferon-γ (IFN-γ). To induce differentiation, podocytes were maintained at 37°C on collagen-coated dishes without IFN-γ to allow differentiation for at least 7 days. Differentiated cells were identified by their large arborized shape and by the cell expression of a known differentiation marker synaptopodin. Differentiated podocytes were cultured for 24 h in RPMI 1640 medium containing 5 mM D-glucose and 1% FCS before being exposed to various experimental conditions. The cells were then divided into four groups: (1) normal glucose group (NG) as controls incubated in RPMI 1640 containing 5 mM glucose, (2) high glucose group (HG) incubated in RPMI 1640 containing 30 mM glucose, (3) EMO group incubated in HG medium treated with 30μg /ml of EMO for 24 h, (4) QLT-0267 group incubated in HG medium treated with 1μmol/ml of ILK inhibitor QLT0267 for 24 h. All the glucose used in the present study was D-glucose. All experimental groups were cultured in quadruplicate. Growth curves and cell viability assay. [1] Metabolic activity (used as a measure of cell viability) was determined using the Alamar Blue® assay run according to instructions provided by the manufacturer. For growth curves, 1,000 cells were seeded in triplicate onto 96-well flat bottom tissue culture plates and allowed to adhere to the substratum for 24 hrs under normal growth conditions (37°C and 5% CO2 in a humidified atmosphere). Thereafter, for 7 days the number of viable cells was estimated by Alamar blue. For cytotoxicity assays, 6,000 cells/well (seeded in triplicate onto 96-well flat bottom tissue culture plates) were allowed to adhere for 24 hrs under normal growth conditions (37°C and 5% CO2 in a humidified atmosphere). Serial dilutions of Dt or vehicle controls diluted in DMEM cell culture medium were added to wells and cells were grown for an additional 72 hrs before measuring cell viability. To assess cell viability, cells were incubated with 10% resazurin solution for 4 hrs at 37°C and fluorescence was measured at 560/590 nm using an Optima fluorescence plate reader. Relative fluorescence determined from drug treated cells was normalized to fluorescence determined from control cells (cells grown in the absence of added drug) and the percent (%) cell viability relative to vehicle control treated cells (100% viability) was determined. Background fluorescence was subtracted from all samples and results of experiments conducted in triplicate at least three times were averaged. |
| Animal Protocol |
For the second study (Fig. 3), mice were randomized into three groups (20 mice per group) according to the site of tumor inoculation (i.p., o.t. or i.c.). Tumors were established as described above and on day 7, animals were treated with 1 dose of 10 mg/kg Dt spiked with tritium labeled Dt. Four animals were removed per group at 0.5, 1, 2, 4 and 24 hrs post treatment, at which time blood was collected via cardiac puncture and organs were harvested as outlined in the methods below. For the third study (Fig. 4), mice were randomized into three groups according to the site of tumor inoculation (i.p., o.t. or i.c.) and further randomized into two sub-groups (six mice per group) (vehicle and 5 mg/kg). Tumors were established as described above and readily detectable by luminescence in all animals 24 hrs and 7 days post-inoculation. On day 7, animals were treated with the vehicle or Dt (5 mg/kg) (i.v. Q7D × 4), a well tolerated Dt dose selected to better differentiate between the drug's activity in the models. Animals were monitored for growth, body weight and survival. Tumor growth was monitored using the IVIS 200. For the fourth study (Fig. 5), mice were randomized into two groups according to the site of tumor inoculation (i.p. or i.c.) and further randomized into four sub-groups (six mice per group) according to the dose of docetaxel (5, 10, 15 mg/kg) used. Tumors were established as described above and on day 7, animals were treated with Dt or the vehicle control (i.v. Q7D × 4). Animals were monitored for growth, body weight and survival. Tumor growth was monitored using the IVIS 200. For the fifth study (Fig. 6) mice were randomized into two groups to be inoculated with LCC6WT-luc cells and further randomized into four sub-groups according to treatment (vehicle, 10 mg/kg Dt, 200 mg/kg QLT0267 or a combination of 10 mg/kg Dt and 200 mg/kg QLT0267) (five mice per group). The QLT0267 dose (200 mg/kg) and schedule (QD × 28) was selected based on previous studies that showed effective therapy in different human xenograft models. tumors were established as described above and on day 7, animals were treated with (vehicle; Dt-10 mg/kg [i.v.] [Q7D × 4]; QLT0267-200 mg/kg [p.o.] [QD × 28]; or a combination of Dt-10 mg/kg [i.v.] [Q7D × 4] and QLT0267-200 mg/kg [p.o.] [QD × 28]). Animals were monitored for tumor growth (IVIS 200), body weight and overall health status. [1] Healthy male Sprague-Dawley rats weighing 200 to 220g, were housed in an air-conditioned room at 23 ± 1°C on a 12:12-h light-dark cycle. Animals were fed a standard diet and given water ad-libitum. Diabetes was induced in rats by a single intraperitoneal injection of streptozotocin (STZ) dissolved in 0.1M citrate buffer (pH 4.5) at 60 mg/kg, while the normal control rats received the 0.1M citrate buffer solution.72 hours after injection of STZ, the blood glucose level was measured from the tail vein. Rats with a blood glucose level over 300 mg/dl were considered to be diabetic and included in the study. Diabetic rats were then randomly divided into four groups (n=12/each group): (1) STZ-induced diabetic rats (DM); (2) STZ-induced diabetic rats treated with a dose of EMO at 20 mg· kg-1· d-1 (DM+EMO);(3) STZ-induced diabetic rats treated with a dose of ILK inhibitor,QLT0267 at 10 mg· kg-1· d-1 (DM+QLT0267); (4) Normal Sprague-Dawley rats were choosed as control (Normal). EMO and QLT0267 were started at 2 weeks after STZ injection and were administered once daily by oral garage for 12 weeks. The normal control (Normal) and diabetic control (DM) rats were treated with the same volume of saline within the same time. At the end of the 12-week treatment, animals were then killed and the kidneys were harvested immediately. [3] |
| References |
[1]. Validating the Use of a Luciferase Labeled Breast Cancer Cell Line, MDA435LCC6, as a Means to Monitor Tumor Progression and to Assess the Therapeutic Activity of an Established Anticancer Drug, Docetaxel (Dt) Alone or in Combination With the ILK Inhibitor, QLT0267. Cancer Biol Ther. 2011 May 1;11(9):826-38. [2]. A facile consensus ranking approach enhances virtual screening robustness and identifies a cell-active DYRK1α inhibitor. Future Med Chem. 2018 Oct;10(20):2411-2430. [3]. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell Physiol Biochem. 2015;35(4):1425-36. |
| Additional Infomation |
A significant issue in drug efficacy studies is animal study design. Here we hypothesize that when evaluating new or existing therapeutics for the treatment of cancer, the location of disease burden will influence drug efficacy. To study this, Female NCr nude mice were inoculated with luciferase-positive human breast cancer cells (LCC6WT-luc) orthotopically (o.t.), intraperitoneally (i.p.) or intracardiacly (i.c.) to create localized, ascites or disseminated disease, respectively. Tumor development was monitored using bioluminescence imaging. Docetaxel (Dt) pharmacokinetics and distribution to sites of tumor growth were determined. Disease progression was followed in animals treated with Dt alone and in combination with QLT0267, an Integrin Linked Kinase inhibitor. Tumor related morbidity was most rapid when cells were inoculated i.c., where disease progression was observed in brain, ovaries, adrenal glands, and lungs. Dt pharmacokinetics were comparable regardless of the model used (mean plasma AUC0-24 hrs 482.6 ng/ml*hr), however, Dt levels were lowest in those tissues developing disease following i.c. cell injection. Treatment with low dose Dt (5 mg/kg) increased overall survival and reduced tumor cell growth in all three models but the activity was greatest in mice with orthotopic tumors. Higher doses of Dt (15 mg/kg) was able to prolong survival in animals bearing i.p. tumors but not i.c. tumors. Addition of QLT0267 provided no added benefit above Dt alone in the disseminated model. These studies highlight a need for more comprehensive in vivo efficacy studies designed to assess multiple disease models and multiple endpoints, focusing analysis of drug parameters on the most chemoresistant disease. [1] Background: Virtual screening is vital for contemporary drug discovery but striking performance fluctuations are commonly encountered, thus hampering error-free use. Results and Methodology: A conceptual framework is suggested for combining screening algorithms characterized by orthogonality (docking-scoring calculations, 3D shape similarity, 2D fingerprint similarity) into a simple, efficient and expansible python-based consensus ranking scheme. An original experimental dataset is created for comparing individual screening methods versus the novel approach. Its utilization leads to identification and phosphoproteomic evaluation of a cell-active DYRK1α inhibitor. Conclusion: Consensus ranking considerably stabilizes screening performance at reasonable computational cost, whereas individual screens are heavily dependent on calculation settings. Results indicate that the novel approach, currently available as a free online tool, is highly suitable for prospective screening by nonexperts. [2] Background: Epithelial-to-mesenchymal transition (EMT) is a potential pathway leading to podocyte depletion and proteinuria in diabetic kidney disease (DKD). Here, we investigated the protective effects of Emodin (EMO) on high glucose (HG) induced-podocyte EMT in-vitro and in-vivo. Methods: Conditionally immortalized mouse podocytes were exposed to HG with 30 μg /ml of EMO and 1 μmol/ml of integrin-linked kinase (ILK) inhibitor QLT0267 for 24 h. Streptozotocin (STZ)-induced diabetic rats were treated with EMO at 20 mg· kg(-1)· d(-1) and QLT0267 at 10 mg· kg(-1)· w(-1) p.o., for 12 weeks. Albuminuria and blood glucose level were measured. Immunohistochemistry, immunofluorescence, western blotting and real-time PCR were used to detect expression of ILK, the epithelial marker of nephrin and the mesenchymal marker of desmin in-vitro and in-vivo. Results: HG increased podocyte ILK and desmin expression while decreased nephrin expression. However, EMO significantly inhibited ILK and desmin expression and partially restored nephrin expression in HG-stimulated podocytes. These in-vitro observations were further confirmed in-vivo. Treatment with EMO for 12 weeks attenuated albuminuria, renal histopathology and podocyte foot process effacement in diabetic rats. EMO also repressed renal ILK and desmin expression, preserved nephrin expression, as well as ameliorated albuminuria in STZ-induced diabetic rats. Conclusion: EMO ameliorated glucose-induced EMT and subsequent podocyte dysfunction partly through ILK and desmin inhibition as well as nephrin upregulatiotion, which might provide a potential novel therapeutic option for DKD. [3] |
Solubility Data
| Solubility (In Vitro) | DMSO : ~83.33 mg/mL (~358.81 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (8.96 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (8.96 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.3059 mL | 21.5295 mL | 43.0589 mL | |
| 5 mM | 0.8612 mL | 4.3059 mL | 8.6118 mL | |
| 10 mM | 0.4306 mL | 2.1529 mL | 4.3059 mL |