Proxyphylline is a derivative of methylxanthine that is clinically used as a cardiac stimulant, a vasodilator and a bronchodilator. Proxyphylline acts by specifically opposing A2 adenosine receptors (Ki = 850 µM for platelets) in contrast to A1 adenosine receptors (Ki of 82 nM for the bovine brain). Vasodilatory and cardiac stimulatory effects have been observed with proxyphylline. It causes a clear positive inotropic effect along with an increase in coronary flow. Half-maximum relaxation of tracheal smooth muscle is achieved with 100 μg/mL of proxyphylline, which suppresses tracheal PDE-activity.
Physicochemical Properties
| Molecular Formula | C10H14N4O3 | |
| Molecular Weight | 238.25 | |
| Exact Mass | 238.107 | |
| Elemental Analysis | C, 50.41; H, 5.92; N, 23.52; O, 20.15 | |
| CAS # | 603-00-9 | |
| Related CAS # |
|
|
| PubChem CID | 4977 | |
| Appearance | White to off-white solid powder | |
| Density | 1.46 g/cm3 | |
| Boiling Point | 487.2ºC | |
| Melting Point | 134-136ºC | |
| Flash Point | 248.5ºC | |
| Index of Refraction | 1.664 | |
| LogP | -0.8 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 4 | |
| Rotatable Bond Count | 2 | |
| Heavy Atom Count | 17 | |
| Complexity | 348 | |
| Defined Atom Stereocenter Count | 0 | |
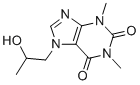
| SMILES | O([H])C([H])(C([H])([H])[H])C([H])([H])N1C([H])=NC2=C1C(N(C([H])([H])[H])C(N2C([H])([H])[H])=O)=O |
|
| InChi Key | KYHQZNGJUGFTGR-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C10H14N4O3/c1-6(15)4-14-5-11-8-7(14)9(16)13(3)10(17)12(8)2/h5-6,15H,4H2,1-3H3 | |
| Chemical Name | 7-(2-hydroxypropyl)-1,3-dimethylpurine-2,6-dione | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | A1 adenosine receptor | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| References |
[1]. Effects of aminophylline, proxyphylline and a proxyphylline-Melilotus extract-rutin mixture(theoesberiven) on the heart and the coronary circulation. Jpn J Pharmacol. 1977 Oct;27(5):709-20. [2]. Overadditive synergism between theophylline, diprophylline and proxyphylline in tracheal smooth muscle relaxation. Arzneimittelforschung. 1983;33(10):1450-4. [3]. Pharmacokinetics and bronchodilatory effect of proxyphylline and theophylline. Eur J Respir Dis. 1984 Jan;65(1):20-7. [4]. Inhibition of human lung cyclic nucleotide phosphodiesterases by proxyphylline, theophylline and their metabolites. Acta Pharmacol Toxicol (Copenh). 1982 Sep;51(3):250-2. |
||
| Additional Infomation |
Proxyphylline is an oxopurine. Proxyphylline is a methylxanthine and derivative of theophylline. Proxyphylline relaxes smooth muscles, particularly bronchial muscles. This xanthine most likely exerts its effect by inhibiting cAMP or cGMP phosphodiesterases, thereby increasing levels of the second messenger cAMP or cGMP intracellularly. Other mode of actions include an adenosine antagonistic effect on the activity of CD4 lymphocytes and mediator release from mast cells thereby decreasing lung sensitivity to allergens and other substances that cause inflammation. Proxyphylline also acts as a CNS stimulant and exerts a positive chronotropic and inotropic effect on the heart. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (10.49 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (10.49 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (10.49 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1973 mL | 20.9864 mL | 41.9727 mL | |
| 5 mM | 0.8395 mL | 4.1973 mL | 8.3945 mL | |
| 10 mM | 0.4197 mL | 2.0986 mL | 4.1973 mL |