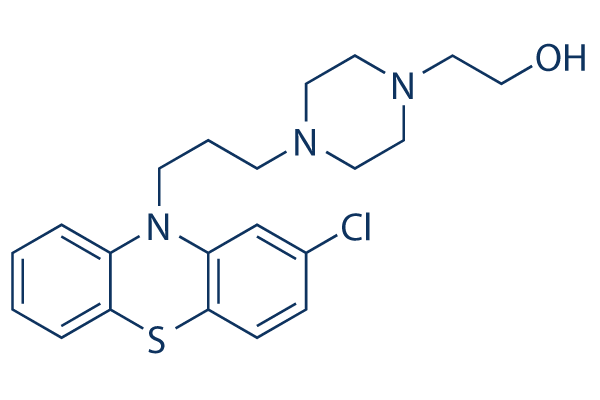
Perphenazine (also known as Perphenazin, Trilafon, Etaperazine) is a phenothiazine derivative and a dopamine antagonist with antiemetic and antipsychotic properties. Perphenazine has a somewhat strong potency and primarily blocks dopamine 2 (D2) receptors, but it may also have antagonistic effects at histamine 1 (H1), cholinergic M1, and alpha 1 adrenergic receptors in the vomiting center, which would lessen nausea and vomiting. Treatment for specific mental/emotional disorders (e.g., schizophrenia, manic phase of bipolar disorder, schizoaffective disorder) has been implemented.
Physicochemical Properties
| Molecular Formula | C21H26CLN3OS | |
| Molecular Weight | 403.97 | |
| Exact Mass | 403.148 | |
| Elemental Analysis | C, 62.44; H, 6.49; Cl, 8.78; N, 10.40; O, 3.96; S, 7.94 | |
| CAS # | 58-39-9 | |
| Related CAS # | Perphenazine-d4; 155593-75-2; Perphenazine dihydrochloride; 2015-28-3; Perphenazine-d8 dihydrochloride; 2070015-23-3 | |
| PubChem CID | 4748 | |
| Appearance | White to off-white crystalline powder | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 580.4±50.0 °C at 760 mmHg | |
| Melting Point | 35339ºC | |
| Flash Point | 304.8±30.1 °C | |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C | |
| Index of Refraction | 1.627 | |
| LogP | 4.34 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 5 | |
| Rotatable Bond Count | 6 | |
| Heavy Atom Count | 27 | |
| Complexity | 463 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | ClC1C([H])=C([H])C2=C(C=1[H])N(C1=C([H])C([H])=C([H])C([H])=C1S2)C([H])([H])C([H])([H])C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])O[H])C([H])([H])C1([H])[H] |
|
| InChi Key | RGCVKNLCSQQDEP-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C21H26ClN3OS/c22-17-6-7-21-19(16-17)25(18-4-1-2-5-20(18)27-21)9-3-8-23-10-12-24(13-11-23)14-15-26/h1-2,4-7,16,26H,3,8-15H2 | |
| Chemical Name | 2-[4-[3-(2-chlorophenothiazin-10-yl)propyl]piperazin-1-yl]ethanol | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | D2 Receptor ( Ki = 0.56 nM ); D3 Receptor ( Ki = 0.43 nM ); D4 Receptor ( Ki = 28.5 nM ); 5-HT2A Receptor ( Ki = 5.6 nM ); 5-HT6 Receptor ( Ki = 17 nM ); 5-HT7 Receptor ( Ki = 23 nM ); H2 Receptor ( Ki = 132 nM ); 5-HT1A Receptor ( Ki = 421 nM ) | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | Perphenazine Is a common antipsychotic medication that inhibits the α1A adrenergic receptor, the 5-HT2A receptor, the D2/D3 dopamine receptor, the D2L receptor, and the histamine H1 receptor (H1) with Ki values of 5.6, 10, 0.765/0.13, 3.4, and 8 nM. Ki, 5-HT2A: 5.6 nM; Ki,α1A: 10 nM; Ki,D2/D3): 0.765/0.13 nM; Ki,D2L receptor: 3.4 nM; Ki,H1: 8 nM. As for the IC50 values, they are as follows. | ||
| Cell Assay | On 96-well plates, cells are plated, and medications are applied for varied lengths of time. After that, the cells are incubated for one hour with the MTS assay reagent. After that, a microplate reader is used to read the plates at 490 nm. | ||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Absolute bioavailability is 40% following oral administration. Perphenazine is extensively metabolized in the liver to a number of metabolites by sulfoxidation, hydroxylation, dealkylation, and glucuronidation. Phenothiazines are generally well absorbed from the GI tract and from parenteral sites; however, absorption may be erratic, particularly following oral administration. Considerable interindividual variations in peak plasma concentrations have been reported. The variations in peak plasma concentrations may result from genetic differences in the rate of metabolism, biodegradation of the drug in the GI lumen, and/or metabolism of the drug during absorption (in the GI mucosa) and first pass through the liver. /Phenothiazine General Statement/ Following oral administration of perphenazine tablets, mean peak plasma perphenazine concentrations were observed between 1 to 3 hours. ... In a study in which normal volunteers (n=12) received perphenazine 4 mg q8h for 5 days, steady-state concentrations of perphenazine were reached within 72 hours. Phenothiazines and their metabolites are distributed into most body tissues and fluids, with high concentrations being distributed into the brain, lungs, liver, kidneys, and spleen. /Phenothiazine General Statement/ Phenothiazines are highly bound to plasma proteins. /Phenothiazine General Statement/ For more Absorption, Distribution and Excretion (Complete) data for Perphenazine (10 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Perphenazine is extensively metabolized in the liver to a number of metabolites by sulfoxidation, hydroxylation, dealkylation, and glucuronidation. Most metabolites of phenothiazines are pharmacologically inactive; however, certain metabolites (eg, 7-hydroxychlorpromazine, mesoridazine) show moderate pharmacologic activity and may contribute to the action of the drugs. There is limited evidence to indicate that some phenothiazines (eg, chlorpromazine) may induce their own metabolism. /Phenothiazine General Statement/ The pharmacokinetics of a single oral dose of 6 mg perphenazine was studied in a group of six slow and six rapid hydroxylators of debrisoquin. Peak serum concentrations of perphenazine were significantly higher in slow hydroxylators than they were in rapid hydroxylators (2.4 +/- 0.6 versus 0.7 +/- 0.3 nmol/L, p less than 0.001). The AUC(0-12) was also higher in slow hydroxylators than it was in rapid hydroxylators (18.5 +/- 6.2 versus 4.5 +/- 2.5 nmol.L-1.hr, p less than 0.001). The data suggest that the disposition of the antipsychotic drug perphenazine covaries with polymorphic debrisoquin hydroxylation. After chronic administration of piperazine-substituted phenothiazine drugs ... to rats, tissues contained drug metabolites, in which piperazine ring fission by multiple oxidative n-dealkylation had occurred to give substituted ethylenediamine. Thus, n-[gamma-(2-chlorophenothiazinyl-10)-propyl]ethylenediamine ... from ... perphenazine ... Perphenazine has known human metabolites that include Perphenazine sulfoxide and N-Dealkylated perphenazine. Hepatic. Route of Elimination: Perphenazine is extensively metabolized in the liver to a number of metabolites by sulfoxidation, hydroxylation, dealkylation, and glucuronidation. Half Life: 8-12 hours, but ranges up to 20 hours. Biological Half-Life 8-12 hours, but ranges up to 20 hours. The plasma elimination half-life of perphenazine was independent of dose and ranged between 9 and 12 hours. ...The average terminal half-life of PPZ was approximately 9.5 hours. ... Peak 7-hydroxyperphenazine concentrations were observed between 2 to 4 hours with a terminal phase half-life ranging between 9.9 to 18.8 hours. |
||
| Toxicity/Toxicokinetics |
Toxicity Summary Binds to the dopamine D1 and dopamine D2 receptors and inhibits their activity. The mechanism of the anti-emetic effect is due predominantly to blockage of the dopamine D2 neurotransmitter receptors in the chemoreceptor trigger zone and vomiting centre. Perphenazine also binds the alpha andrenergic receptor. This receptor's action is mediated by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Toxicity Data LD50:318 mg/kg (Oral, Rat) (A308) LD50: 64 mg/kg (Intraperitoneal, Mouse) (A308) Interactions Since phenothiazines and central nervous system depressants (opiates, analgesics, antihistamines, barbiturates) can potentiate each other, less than the usual dosage of the added drug is recommended and caution is advised when they are administered concomitantly. Perphenazine ... increased t1/2 of amphetamine in brain. However, despite elevated amphetamine levels, amphetamine-induced pharmacological effects were inhibited. It appears that whether given im or orally, antiparkinsonism drugs biperidine & orphenadine have no significant effect on previously attained levels of perphenazine and its metabolites in psychotic pt. QT interval-prolonging medications, including cisapride, erythromycin, and quinidine /may produce/ additive QT interval prolongation increasing the risk of developing cardiac arrhythmias when /concurrently administered with phenothiazines/. /Phenothiazines/ For more Interactions (Complete) data for Perphenazine (31 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat oral 318 mg/kg LD50 Rat ip 146 mg/kg LD50 Rat iv 34 mg/kg LD50 Mouse oral 120 mg/kg For more Non-Human Toxicity Values (Complete) data for Perphenazine (7 total), please visit the HSDB record page. |
||
| References |
[1]. Annals od palliative medicine. 2012, 1(2):137-142. [2]. Bosn J Basic Med Sci . 2013 May;13(2):119-25. [3]. Acta Vet. Brno. 2011, 80: 87-92. [4]. Animal Cells and Systems. 2012, 16(1):20-26. |
||
| Additional Infomation |
Therapeutic Uses Antipsychotic Agents, Phenothiazine; Dopamine Antagonists Perphenazine is indicated for use in the treatment of schizophrenia and for the control of severe nausea and vomiting in adults. /Included in US product label/ Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation. /Included in US product label/ The US Food and Drug Administration (FDA) currently advises clinicians that antipsychotic agents are not approved for the treatment of dementia-related psychosis. FDA further advises clinicians that no drugs currently are approved for the treatment of patients with dementia-associated psychosis and that other management options should be considered in such patients. /Phenothiazine General Statement/ VET: ...To help control intractable animals, relieve pain, control motion sickness, and as preanesthetic agent. Drug Warnings /BOXED WARNING/ WARNING: Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine is not approved for the treatment of patients with dementia-related psychosis. ... Extrapyramidal reactions ... fairly common, usually 3 types ... Parkinsonian-like syndrome ... dystonia and dyskinesia, including torticollis, tics, and other involuntary muscle movements ... akathisia, shown by restlessness ... hyperreflexia, reported in newborn ... ./Phenothiazines/ Perphenazine products are contraindicated in comatose or greatly obtunded patients and in patients receiving large doses of central nervous system depressants (barbiturates, alcohol, narcotics, analgesics, or antihistamines); in the presence of existing blood dyscrasias, bone marrow depression, or liver damage; and in patients who have shown hypersensitivity to perphenazine tablets, their components, or related compounds. Perphenazine products are also contraindicated in patients with suspected or established subcortical brain damage, with or without hypothalamic damage, since a hyperthermic reaction with temperatures in excess of 104 °F may occur in such patients, sometimes not until 14 to 16 hours after drug administration. Total body ice-packing is recommended for such a reaction; antipyretics may also be useful. For more Drug Warnings (Complete) data for Perphenazine (47 total), please visit the HSDB record page. Pharmacodynamics Perphenazine is a piperazinyl phenothiazine, acts on the central nervous system, and has a greater behavioral potency than other phenothiazine derivatives whose side chains do not contain a piperazine moiety. It is a member of a class of drugs called phenothiazines, which are dopamine D1/D2 receptor antagonists. Perphenazine is 10 to 15 times as potent as chlorpromazine; that means perphenazine is a highly potent antipsychotic. In equivalent doses it has approximately the same frequency and severity of early and late extrapypramidal side-effects compared to Haloperidol. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.19 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (6.19 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.19 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4754 mL | 12.3772 mL | 24.7543 mL | |
| 5 mM | 0.4951 mL | 2.4754 mL | 4.9509 mL | |
| 10 mM | 0.2475 mL | 1.2377 mL | 2.4754 mL |