Physicochemical Properties
| Molecular Formula | C27H30O15 |
| Molecular Weight | 594.5181 |
| Exact Mass | 594.158 |
| Elemental Analysis | C, 54.55; H, 5.09; O, 40.37 |
| CAS # | 114482-86-9 |
| Related CAS # | 114482-86-9 |
| PubChem CID | 10077207 |
| Appearance | Light yellow to yellow solid |
| Density | 1.8±0.1 g/cm3 |
| Boiling Point | 957.5±65.0 °C at 760 mmHg |
| Flash Point | 318.1±27.8 °C |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.745 |
| LogP | -0.57 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 42 |
| Complexity | 958 |
| Defined Atom Stereocenter Count | 10 |
| SMILES | O1[C@]([H])([C@@]([H])([C@]([H])([C@@]([H])([C@@]1([H])C([H])([H])O[C@@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])O[H])O[H])O[H])O[H])O[H])OC1C([H])=C2C(C(C([H])=C(C3C([H])=C([H])C([H])=C([H])C=3[H])O2)=O)=C(C=1O[H])O[H] |
| InChi Key | HAYLVXFWJCKKDW-IJTBWITGSA-N |
| InChi Code | InChI=1S/C27H30O15/c28-8-15-19(31)22(34)24(36)26(41-15)38-9-16-20(32)23(35)25(37)27(42-16)40-14-7-13-17(21(33)18(14)30)11(29)6-12(39-13)10-4-2-1-3-5-10/h1-7,15-16,19-20,22-28,30-37H,8-9H2/t15-,16-,19-,20-,22+,23+,24-,25-,26-,27-/m1/s1 |
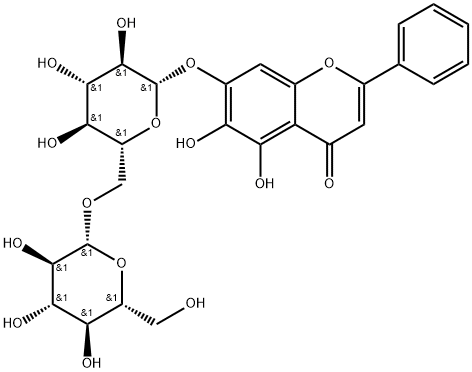
| Chemical Name | 5,6-dihydroxy-2-phenyl-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| Synonyms | Oroxin B; 114482-86-9; Baicalin-7-diglucoside; Baicalein 7-O-diglucoside; DTXSID501312120; 5,6-dihydroxy-2-phenyl-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one; MFCD22125002; Oroxin B (Standard) |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PTEN; COX-2; PI3K; Akt |
| ln Vitro |
Oroxin B (0-2 μM, 48 hours) causes the human TRAY cell line (SMMC 7721) to glow during proliferation (48 hours) and induce cell filling (12 hours) [1]. In SMMC 7721, oroxin B (0-2 μM, 48) increases PTEN and suppresses p-AKT, COX-2, VEGF, and PI3K[1]. In Raji cells, Oroxin B (160 μM, 24 hours) reduced IL-1β-induced inflammation-related indicators (iNOS, COX-2, TNF-α, IL-6: rhodamine-labeled glyburin arteries) and torsion-induced ER stress hormones (dorsal markers)[2]. The results showed that Oroxin B/OB inhibited proliferation of SMMC-7721 cell in a dose-dependent manner, and induced its apoptosis. Moreover, OB unregulated PTEN and downregulated COX-2, VEGF, p-AKT, and PI3K. Conclusion: Our results demonstrated that Oroxin B/OB significantly inhibits proliferation and induce apoptosis, which may be strongly associated with the inhibiting COX-2/VEGF and PTEN/PI3K/AKT pathway signaling pathway in SMMC-7721 cells, OB potentially be used as a novel therapeutic agent for liver cancer. Summary: OB (Oroxin B) is one of the effective flavonoid components of traditional Chinese medicine O. indicum (L.)OB can inhibite the proliferation and promoted apoptosis of the human hepatoma cell line SMMC 7721OB plays a role of antitumor effect may to regulate COX 2/VEGF and PTEN/PI3K/AKT pathways directly or indirectly. Abbreviations used: OB: Oroxin B; MTT: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; COX-2: cyclooxygenase-2; PI3K: phosphatidylinositol 3 kinase; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; VEGF: Vascular endothelial growth factor; RT-PCR: Reverse transcription polymerase chain reaction; DAPI: Diamidino 2 phenylindole; PBS: Phosphate buffer saline; FITC: Fluorescein isothiocyanate; PI: Propidium Iodide; RIPA: Radio immunoprecipitation assay lysis buffer; PMSF: Phenylmethanesulfonyl fluoride; PAGE: Polyacrylamide gel electrophoresis. [1] In this study, we explored unique tumor-suppressive ER stress agents from the traditional Chinese medicinal herb Oroxylum indicum, and found that a small molecule Oroxin B selectively induced tumor-suppressive ER stress in malignant lymphoma cells, but not in normal cells, effectively inhibited lymphoma growth in vivo, and significantly prolonged overall survival of lymphoma-xenografted mice without obvious toxicity. Mechanistic studies have revealed that the expression of key tumor-adaptive ER-stress gene GRP78 was notably suppressed by Oroxin B via down-regulation of up-stream key signaling protein ATF6, while tumor-suppressive ER stress master gene DDIT3 was strikingly activated through activating the MKK3-p38 signaling pathway, correcting the imbalance between tumor-suppressive DDIT3 and tumor-adaptive GRP78 in lymphoma. [2] OB reversed the expression level of anabolic-related proteins (Aggrecan and Collagen II) and catabolic-related (MMP3, MMP13, and ADAMTS5) in IL-1β-induced chondrocytes. Mechanistically, OB suppressed the inflammatory response stimulated by IL-1β, as the inflammation-related (iNOS, COX-2, TNF-α, IL-6, and IL-1β) markers were downregulated after the administration of OB in IL-1β-induced chondrocytes. Besides, the activation of PI3K/AKT/mTOR signaling pathway induced by IL-1β could be inhibited by OB. Additionally, the autophagy process impaired by IL-1β could be rescued by OB. What's more, the introduction of 3-MA to specifically inhibit the autophagic process impairs the protective effect of OB on cartilage. [3] |
| ln Vivo |
In the human simulated cell (Raji Cell) xenograft model, oroxin B (30 mg/kg, intraperitoneal injection, 28 days) can stimulate the middle part of the ER in simulated cells and prevent tumor growth [2]. When knee OA is caused by medial meniscus (DMM), oroxin B (160 μM, 10 μL) injection into the probing knee joint can preserve the unstable articular cartilage [3]. For HFD feeding, Oroxin B (200 mg/kg/day, side gavage) may be utilized.
Oroxin B effectively inhibits lymphoma growth and significantly prolongs overall survival of tumor-bearing mice without toxicity [2] In light of that the anti-tumor function of Oroxin B have not been reported yet, oroxin B selectively induces malignant lymphoma cell ER stress and inhibits tumor cell growth, we evaluated anti-lymphoma effect of oroxin B in vivo using human lymphoma cell xenograft model with two different oroxin B treatment sets. In the first set of treatment experiments, the human malignant lymphoma Raji cell-xenografted SCID mice were treated with oroxin B on the first day of the xenograft and continued for 28 days with daily administration of oroxin B at the dose of 30 mg/kg. After 28 days of treatment, the average tumor weights of the oroxin B-treated and saline control mice were 162.8 ± 20 mg and 665.7 ± 140 mg (Fig. 2A and B), respectively, the average tumor volumes were 82.1 ± 33 mm3 and 1327.3 ± 2.5 mm3 (Fig. 2C), respectively, and the total body weight and organ index between the oroxin treatment and saline control groups were not different (Supplementary Fig. S3A and B). Additionally, oroxin B treatment did not significantly affect all of the 12 hematological indicators tested, including the white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (HGB) level, and platelet (PLT) count (Supplementary Table 3). In addition, WBC and PLT counts were moderately increased after oroxin B treatment, although the difference was not significant. Collectively, theses in vivo data indicate that Oroxin B exerts an anti-lymphoma effect without toxicity at the doses tested. OB/Oroxin B ameliorated cartilage destruction in DMM–induced mice OA model [3] The in vivo experiment was to explore the effect of OB/Oroxin B in mice knee cartilage. The DMM method was employed to establish the OA cartilage model. H&E and SOFG staining showed that the DMM group characterized by OA features, such as unevenness, thinning, and cracking of the cartilage surface, and reduction of chondrocytes compared with the SHAM group and the SHAM + OB group. However, we observed a chondroprotective effect of OB in the DMM + OB group, as cartilage damage was alleviated after the OB treatment ( Figures 8A, B ). Besides, the chondroprotective effect of OB was verified by the OARSI score system which reflected the severity of cartilage damage ( Figure 8C ). In addition, the detection of key proteins of anabolism and catabolism in chondrocytes was also performed in vivo experiments. IHC staining ( Figures 8D, E ) exhibited a decreased expression of anabolic-related proteins (Aggrecan, Collagen II) and an increased expression of catabolic-related protein (MMP13) in the DMM group compared with the SHAM group and the SHAM + OB group, while the changes were reversed in the DMM + OB group, which was consistent with results illustrated in vitro experiment. Collectively, the above data proved that OB prosses the property of protecting articular cartilage in DMM-induced OA in vivo. Metabolic-associated fatty liver disease (MAFLD) has become a common chronic liver disease, but there is no FDA-approved drug for MAFLD treatment. Numerous studies have revealed that gut microbiota dysbiosis exerts a crucial effect on MAFLD progression. Oroxin B is a constituent of the traditional Chinese medicine Oroxylum indicum (L.) Kurz. (O. indicum), which has the characteristics of low oral bioavailability but high bioactivity. However, the mechanism through which oroxin B improves MAFLD by restoring the gut microbiota balance remains unclear. To this end, we assessed the anti-MAFLD effect of Oroxin B in HFD-fed rats and investigated the underlying mechanism. Our results indicated that oroxin B administration reduced the lipid levels in the plasma and liver and lowered the lipopolysaccharide (LPS), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α) levels in the plasma. Moreover, oroxin B alleviated hepatic inflammation and fibrosis. Mechanistically, oroxin B modulated the gut microbiota structure in HFD-fed rats by increasing the levels of Lactobacillus, Staphylococcus, and Eubacterium and decreasing the levels of Tomitella, Bilophila, Acetanaerobacterium, and Faecalibaculum. Furthermore, oroxin B not only suppressed Toll-like receptor 4-inhibitor kappa B-nuclear factor kappa-B-interleukin 6/tumor necrosis factor-α (TLR4-IκB-NF-κB-IL-6/TNF-α) signal transduction but also strengthened the intestinal barrier by elevating the expression of zonula occludens 1 (ZO-1) and zonula occludens 2 (ZO-2). In summary, these results demonstrate that oroxin B could alleviate hepatic inflammation and MAFLD progression by regulating the gut microbiota balance and strengthening the intestinal barrier. Hence, our study suggests that oroxin B is a promising effective compound for MAFLD treatment [4]. |
| Cell Assay |
RT-PCR[2] Cell Types: Raji cells Tested Concentrations: 0-40 μM Incubation Duration: 48 h Experimental Results: ER stress diminished mRNA levels of major genes (GRP78 and ATF6. and IL-1β) [3]. MTT assay [1] Cell viability was determined through MTT assays. Briefly, cells were plated in 96-well plates at a density of 1 × 104 per well. After overnight culture, different concentrations of OB/Oroxin B (0.34, 1.01, 1.68 μM) were added to the wells and cells were incubated for 48 h. After drug treatment, the culture medium was replaced with MTT fresh serum-free medium (final concentration, 2.5 mg/ml), followed by a 4 h incubation. In addition, 150 μl of dimethyl sulfoxide was added to each well. Absorbance was measured with a Spectra Max Plus microplate reader at a wavelength of 492 nm. Cell apoptosis [1] The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed according to the manufacturer's instructions. The cells were treated with OB/Oroxin B for 12 h, then 1% paraformaldehyde in PBS fixed 30 min, 0.2% Triton X-100 in PBS permeabilized for 5 min, rinsed with PBS, and incubated with PE buffer (10 mM Tris–HCl, pH 7.5, 80% ethanol) at 37°C for 1 h. Then, they were stained with 4',6-diamidino-2-phenylindole at 37°C for 5 min. Finally, fluorescence was recorded on an inverted fluorescence microscope. The flow cytometer was used FITC/PI double-staining method, each group cells including treated cells (cells were treated with OB/Oroxin B for 48 h) were collected and rinsed twice with cold PBS, mixed with 500 μl of 1 × binding buffer (1 × 106/ml), 5 μl Annexin V-FITC, 5 μl propidium iodide, and incubated in the dark for 15 min and finally sent to the BD Accuri C6 flow cytometer to analyze the cell apoptosis. Cell proliferation assay and cell morphology examination [2] Cell proliferation was determined by the AlamarBlue assay as reported previously by us (Bao et al., 2012). Briefly, cells were cultured in a 96-well plate with either dimethyl sulfoxide as a control or Oroxin B at different concentrations. After incubation for 48 h, 10 μL of AlamarBlue solution was added to each well, and the absorbance at 560 nm and 590 nm was measured using a SpectraMax M5 multi-detection reader. Cell morphology changes were examined by Wright–Giemsa staining (Cao et al., 2013). Colony formation assay [2] Raji cells were incubated with various concentrations of Oroxin B for 48 h and washed in fresh medium. Next, 600 living cells were mixed with Methocult H4230 methylcellulose medium in the absence of oroxin B, and then were plated in a 30-mm plastic dish. After 14 days, colonies in the dish were counted using a dissecting microscope, imaged, and analyzed as previously described (Shang et al., 2014). ER stress assay [2] Raji cells were transfected with the pDsred2-ER vector by electroporation, and DsRed2-positive cells were sorted by flow cytometry. The cells were incubated for 48 h with various concentrations of Oroxin B and transferred onto slides, followed by imaging using Olympus FSX100 microscopy. Cell cycle analysis [2] Raji cells were grown in T75 flasks and treated with Oroxin B at the indicated concentration for 48 h. The DNA contents of propidium iodide (PI)-stained cells were analyzed using flow cytometry as we previously described (Shang et al., 2014, Feng et al., 2012). Cell apoptosis assay [2] Apoptotic cells were assayed using an Annexin V-PI staining kit as previously described (Shang et al., 2014, Feng et al., 2012). Briefly, Raji cells were exposed to Oroxin B at the indicated concentrations for 48 h. The cells were harvested, washed, and re-suspended in phosphate-buffered saline (PBS). Apoptotic or necrotic cells were identified by dual-staining with fluorescein isothiocyanate (FITC)-labeled Annexin V and PI and then were analyzed by flow cytometry as we previously described (Shang et al., 2014, Feng et al., 2012). |
| Animal Protocol |
Animal/Disease Models: HFD-fed rats [4] Doses: 200 mg/kg/day Route of Administration: po (oral gavage) Experimental Results: diminished plasma lipids, LPS, IL-6 and TNF-α levels. Inhibits liver fibrosis by reducing collagen deposition. Mouse lymphoma xenografting and treatment [2] Mouse lymphoma xenografting was performed as we reported previously (Cao et al., 2013) and is described in detail in Supplementary Methods 1. Briefly, each mouse was subcutaneously injected with 107 Raji cells in 200 μL on the back, and then intraperitoneally injected daily with either Oroxin B 30 mg/kg or saline as control. The tumors in the mice were collected and analyzed. In the lymphoma treatment experiment, the lymphoma-bearing mice were intraperitoneally injected with either oroxin B at the dose of 40 or 80 mg/kg, or saline daily. The survival and death of the mice were continuously monitored. Mice OA model generation and treatment [3] For in vivo experiments, the destabilized medial meniscus (DMM) method was applied in the right knee joints of mice to establish to the OA model. Thirty-two C57BL/6 male mice of 8 weeks old were conducted under specific pathogen-free grade conditions. The mice were randomly and equally divided into 4 groups, namely the SHAM group (n=8), the SHAM + OB/Oroxin B group (n=8), the DMM group (n=8), and the DMM + OB/Oroxin B group (n=8). In brief, the SHAM and SHAM + OB groups were underwent the sham surgery (only underwent joint capsulotomy), while the DMM and DMM + OB group mice were underwent the DMM operation. One week after the above operations were done, treatment of intra-articular injections of the knee joint twice a week for 8 weeks was performed. Vehicle (30% PEG300, 5% DMSO, and ddH2O) of 10 μl was injected into the knee joints of mice in the SHAM and DMM groups, while OB (160 μM) of 10 μl was applied in the SHAM + OB group and DMM + OB group. In preliminary experiments, we conducted a toxicity test to determine the experimental dose of Oroxin B and found that the administration of 0–2 g/kg Oroxin B for 2 weeks failed to result in the death of rats and that the administration of 3 g/kg oroxin B caused slight behavioral alterations in the rats (data not shown). Therefore, an oroxin dose of 2 g/kg was deemed a safe dose for rats, and 200 mg/kg/d (a dose equal to 1/10 of this safe dose) was selected as the experimental dose. In addition, in our previous study, many flavonoids, such as hesperetin, baicalein, and oroxin A, exerted anti-MAFLD effects when administered at a high dose (200 mg/kg/d), but lower doses (50 or 100 mg/kg/d) often tended to exert weaker effects (Li et al., 2021; Sun et al., 2018, 2020). Hence, a dose of 200 mg/kg/d oroxin B was used in the subsequent animal experiments. Sprague–Dawley (SD) male rats (180–220 g) were used. The rats were adapted for 7 days by being fed a standard diet. Subsequently, the rats were randomly assigned to three groups (n = 6): the normal rats fed a standard diet were administered vehicle (0.5% CMC-Na, NC), and the HFD-fed MAFLD model rats were randomly administered vehicle (HC) or 200 mg/kg/day Oroxin B (MT) by gavage for 12 weeks. Blood glucose levels were measured at the 1st week and 12th week. During the experimental period, food intake and body weights were measured. After 16 h of fasting, the mice were orally administered 2 g/kg glucose. Blood samples were collected from the tail vein, and the blood glucose levels were measured at 0 (FBG, fasting plasma glucose) and 2 h (2 h-PG, 2-h postprandial glucose) using the glucose oxidase method (Sun et al., 2018). Over the last week, all the animals were fasted and had free access to water for 16 h before being euthanized by CO2. Blood samples were collected, and plasma was extracted by centrifugation at 4000 rpm and 4 °C for 5 min. The liver tissue and colons were rapidly harvested, separated and cleaned as described previously (Weigmann et al., 2007). For colon tissue, the intestines of the dead mice were removed, cut into 4–5-cm pieces and placed in ice-cold PBS, and the intestine was cleared of feces by holding the intestine with forceps and flushing with a syringe filled with 1x PBS. The residual mesenteric fat tissue and Peyer's patches were resected. Portions of liver tissue were stored in 10% formalin for histological analysis, and the other portions of liver tissue and colons were frozen in liquid nitrogen for further studies [4]. |
| Toxicity/Toxicokinetics |
Collectively, theses in vivo data indicate that oroxin B exerts an anti-lymphoma effect without toxicity at the doses tested. Taken together, these data indicate that selective induction of a tumor-suppressive ER stress in malignant lymphoma cells by oroxin B results in effective anti-lymphoma therapy without obvious toxicity. [2] |
| References |
[1]. Evidence for the Involvement of COX-2/VEGF and PTEN/Pl3K/AKT Pathway the Mechanism of Oroxin B Treated Liver Cancer. Pharmacogn Mag. 2018 Apr-Jun;14(54):207-213. [2]. Oroxin B selectively induces tumor-suppressive ER stress and concurrently inhibits tumor-adaptive ER stress in B-lymphoma cells for effective anti-lymphoma therapy. Toxicol Appl Pharmacol. 2015 Oct 15;288(2):269-79. [3]. Oroxin B alleviates osteoarthritis through anti-inflammation and inhibition of PI3K/AKT/mTOR signaling pathway and enhancement of autophagy. Front Endocrinol (Lausanne). 2022 Dec 1;13:1060721. [4]. Oroxin B improves metabolic-associated fatty liver disease by alleviating gut microbiota dysbiosis in a high-fat diet-induced rat model. Eur J Pharmacol. 2023 Jul 15;951:175788. |
| Additional Infomation |
Background: Oroxin B (OB) is one of flavonoids isolated from traditional Chinese herbal medicine Oroxylum indicum (L.) Vent. Recent studies suggest that flavonoids have obvious anti-liver tumors effect, but the precise molecular mechanism is still unclear.
Objective: The current study was performed to investigate the antitumor effects of OB on human hepatoma cell line SMMC-772 and explore the part of molecular mechanisms in this process.
Materials and methods: MTT method, terminal deoxynucleotidyl transferase dUTP nick end labeling assay and flow cytometry were utilized to detect the inhibition of proliferation and the apoptosis after treating OB in of SMMC-7721 cells. The mRNA and proteins expressions of COX-2, vascular endothelial growth factor (VEGF), phosphatidylinositol-3-kinase (PI3K), p-AKT, and PTEN were measured by a real-time polymerase chain reaction and Western Blot method.
Results: The results showed that OB inhibited proliferation of SMMC-7721 cell in a dose-dependent manner, and induced its apoptosis. Moreover, OB unregulated PTEN and downregulated COX-2, VEGF, p-AKT, and PI3K. [1] Cancer cells have both tumor-adaptive and -suppressive endoplasmic reticulum (ER) stress machineries that determine cell fate. In malignant tumors including lymphoma, constant activation of tumor-adaptive ER stress and concurrent reduction of tumor-suppressive ER stress favors cancer cell proliferation and tumor growth. Current ER stress-based anti-tumor drugs typically activate both tumor-adaptive and -suppressive ER stresses, resulting in low anti-cancer efficacy; hence, selective induction of tumor-suppressive ER stress and inhibition of tumor-adaptive ER stress are new strategies for novel anti-cancer drug discovery. Thus far, specific tumor-suppressive ER stress therapeutics have remained absent in clinical settings. In this study, we explored unique tumor-suppressive ER stress agents from the traditional Chinese medicinal herb Oroxylum indicum, and found that a small molecule oroxin B selectively induced tumor-suppressive ER stress in malignant lymphoma cells, but not in normal cells, effectively inhibited lymphoma growth in vivo, and significantly prolonged overall survival of lymphoma-xenografted mice without obvious toxicity. Mechanistic studies have revealed that the expression of key tumor-adaptive ER-stress gene GRP78 was notably suppressed by oroxin B via down-regulation of up-stream key signaling protein ATF6, while tumor-suppressive ER stress master gene DDIT3 was strikingly activated through activating the MKK3-p38 signaling pathway, correcting the imbalance between tumor-suppressive DDIT3 and tumor-adaptive GRP78 in lymphoma. Together, selective induction of unique tumor-suppressive ER stress and concurrent inhibition of tumor-adaptive ER stress in malignant lymphoma are new and feasible approaches for novel anti-lymphoma drug discovery and anti-lymphoma therapy. [2] Background: Osteoarthritis (OA) is a common aging-related degenerative joint disease with chronic inflammation as its possible pathogenesis. Oroxin B (OB), a flavonoid isolated from traditional Chinese herbal medicine, possesses anti-inflammation properties which may be involved in regulating the pathogenesis of OA, but its mechanism has not been elucidated. Our study was the first to explore the potential chondroprotective effect and elucidate the underlying mechanism of OB in OA. Methods: In vitro, primary mice chondrocytes were stimulated with IL-1β along with or without the administration of OB or autophagy inhibitor 3-methyladenine (3-MA). Cell viability assay was measured with a cell counting kit-8 (CCK-8). The phenotypes of anabolic-related (Aggrecan and Collagen II), catabolic-related (MMP3, MMP13, and ADAMTS5), inflammation-related (iNOS, COX-2, TNF-α, IL-6, and IL-1β), and markers of related signaling pathways in chondrocytes with different treatment were detected through western blot, RT-qPCR, and immunofluorescent staining. In vivo, the destabilized medial meniscus (DMM) operation was performed to establish the OA mice model. After knee intra-articular injection with OB for 8 weeks, the mice's knee joints were obtained for subsequent histological staining and analysis. Results: OB reversed the expression level of anabolic-related proteins (Aggrecan and Collagen II) and catabolic-related (MMP3, MMP13, and ADAMTS5) in IL-1β-induced chondrocytes. Mechanistically, OB suppressed the inflammatory response stimulated by IL-1β, as the inflammation-related (iNOS, COX-2, TNF-α, IL-6, and IL-1β) markers were downregulated after the administration of OB in IL-1β-induced chondrocytes. Besides, the activation of PI3K/AKT/mTOR signaling pathway induced by IL-1β could be inhibited by OB. Additionally, the autophagy process impaired by IL-1β could be rescued by OB. What's more, the introduction of 3-MA to specifically inhibit the autophagic process impairs the protective effect of OB on cartilage. In vivo, histological staining revealed that intra-articular injection of OB attenuated the cartilage degradation, as well as reversed the expression level of anabolic and catabolic-related proteins such as Aggrecan, Collagen II, and MMP13 induced in DMM-induced OA models. Conclusions: The study verified that OB exhibited the chondroprotective effect by anti-inflammatory, inhibiting the PI3K/AKT/mTOR signaling pathway, and enhancing the autophagy process, indicating that OB might be a promising agent for the treatment of OA. [3] |
Solubility Data
| Solubility (In Vitro) |
DMSO: 100 mg/mL (~168.2 mM) H2O: <0.1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.21 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.21 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6820 mL | 8.4101 mL | 16.8203 mL | |
| 5 mM | 0.3364 mL | 1.6820 mL | 3.3641 mL | |
| 10 mM | 0.1682 mL | 0.8410 mL | 1.6820 mL |