Physicochemical Properties
| Molecular Formula | C22H33NO4 |
| Molecular Weight | 375.509 |
| Exact Mass | 375.24 |
| Elemental Analysis | C, 70.37; H, 8.86; N, 3.73; O, 17.04 |
| CAS # | 143120-46-1 |
| PubChem CID | 11667940 |
| Appearance | White to off-white solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 554.2±50.0 °C at 760 mmHg |
| Flash Point | 289.0±30.1 °C |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Source | Roots of Stemona tuberosa Lour; Stemona japonica; Stemona phyllantha |
| LogP | 2.28 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 27 |
| Complexity | 636 |
| Defined Atom Stereocenter Count | 10 |
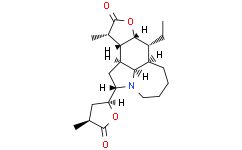
| SMILES | O1C([C@@]([H])(C([H])([H])[H])[C@]2([H])[C@@]1([H])[C@]([H])(C([H])([H])C([H])([H])[H])[C@@]1([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])N3[C@]([H])([C@]4([H])C([H])([H])[C@]([H])(C([H])([H])[H])C(=O)O4)C([H])([H])[C@]2([H])[C@]31[H])=O |
| InChi Key | GYOGHROCTSEKDY-UEIGSNQUSA-N |
| InChi Code | InChI=1S/C22H33NO4/c1-4-13-14-7-5-6-8-23-16(17-9-11(2)21(24)26-17)10-15(19(14)23)18-12(3)22(25)27-20(13)18/h11-20H,4-10H2,1-3H3/t11-,12-,13+,14+,15-,16-,17-,18-,19+,20+/m0/s1 |
| Chemical Name | (2S,7aR,8R,8aR,11S,11aR,11bS,11cR)-8-ethyldodecahydro-11-methyl-2-[(2S,4S)-tetrahydro-4-methyl-5-oxo-2-furanyl]-furo[2,3-h]pyrrolo[3,2,1-jk][1]benzazepin-10(2H)-one |
| Synonyms | Neotuberostemonine; Tuberostemonine LG; CHEBI:69386; (1S,3S,9R,10R,11R,14S,15R,16R)-10-ethyl-14-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]-12-oxa-4-azatetracyclo[7.6.1.04,16.011,15]hexadecan-13-one; CHEMBL479493; SCHEMBL19197265; DTXSID501316122; (+)-Neotuberostemonine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural antitussive alkaloids |
| ln Vitro |
The experiment in vitro showed that NTS significantly reduced the arginase-1 (marker for M2) expression in a dose-dependent manner but down-regulated the iNOS (marker for M1) expression only at 100μM. In conclusion, our study demonstrated for the first time that NTS has a significant protective effect on BLM-induced pulmonary fibrosis through suppressing the recruitment and M2 polarization of macrophages.[1] Neotuberostemonine (NTS) greatly attenuated pulmonary fibrosis induced by bleomycin.[1] NTS significantly relieved macrophage infiltration in bleomycin-induced lung tissue.[1] NTS mainly inhibited the M2, rather than M1, polarization both in vivo and in vitro.[1] |
| ln Vivo |
NTS decreases BLM-induced weight loss, mortality and lung index in mice[1] Administration of BLM in mice is the most commonly model for experimental lung fibrosis. As shown in Fig. 1B, the BLM-injured mice showed significant decrease in the body weight compared with the sham mice. Treatment of these mice with NDN at 40 mg/kg moderately increased the body weight, which was similar with the literature result. NTS treatment at 40 mg/kg (NTS-40) could reduce the body loss induced by BLM during the whole treatment period, and the terminal body weight was... Neotuberostemonine (NTS) is one of the main antitussive alkaloids in the root of Stemona tuberosa Lour. This study aimed to investigate the effects of NTS on bleomycin (BLM)-induced pulmonary fibrosis in mice and the underlying mechanism. After BLM administration, NTS were orally administered to mice at 20 and 40mg/kg per day from days 8 to 21, with nintedanib as a positive control. The effect of NTS on BLM-induced mice was assessed via histopathological examination by HE and Masson's trichrome staining, TGF-β1 level and macrophage recruitment by immunohistochemical staining, expression of profibrotic media and M1/M2 polarization by western blot. RAW 264.7 cells were used to evaluate whether NTS (1, 10, 100μM) directly affected macrophages. The results revealed that NTS treatment significantly ameliorated lung histopathological changes and decreased inflammatory cell counts in the bronchoalveolar lavage fluid. The over-expression of collagen, α-SMA and TGF-β1 was reduced by NTS. Furthermore, NTS markedly lowered the expression of MMP-2 and TIMP-1 while raised the expression of MMP-9. A further analysis showed that NTS was able to decrease the recruitment of macrophages and to inhibit the M2 polarization in mice lung tissues. Pulmonary fibrosis may be partially the result of deregulated tissue repair in response to chronic hypoxia. In this study we explored the effects of hypoxia on lung fibroblasts and the effects of neotuberostemonine (NTS), a natural alkaloid isolated from Stemona tuberosa, on activation of fibroblasts in vitro and in vivo. PLFs (primary mouse lung fibroblasts) were activated and differentiated after exposure to 1% O2 or treatment with CoCl2 (100 μmol/L), evidenced by markedly increased protein or mRNA expression of HIF-1α, TGF-β, FGF2, α-SMA and Col-1α/3α, which was blocked after silencing HIF-1α, suggesting that the activation of fibroblasts was HIF-1α-dependent. NTS (0.1-10 μmol/L) dose-dependently suppressed hypoxia-induced activation and differentiation of PLFs, whereas the inhibitory effect of NTS was abolished by co-treatment with MG132, a proteasome inhibitor. Since prolyl hydroxylation is a critical step in initiation of HIF-1α degradation, we further showed that NTS treatment reversed hypoxia- or CoCl2-induced reduction in expression of prolyl hydroxylated-HIF-1α. With hypoxyprobe immunofiuorescence staining, we showed that NTS treatment directly reversed the lower oxygen tension in hypoxia-exposed PLFs. In a mouse model of lung fibrosis, oral administration of NTS (30 mg·kg-1·d-1, for 1 or 2 weeks) effectively attenuated bleomycin-induced pulmonary fibrosis by inhibiting the levels of HIF-1α and its downstream profibrotic factors (TGF-β, FGF2 and α-SMA). Taken together, these results demonstrate that NTS inhibits the protein expression of HIF-1α and its downstream factors TGF-β, FGF2 and α-SMA both in hypoxia-exposed fibroblasts and in lung tissues of BLM-treated mice. NTS with anti-HIF-1α activity may be a promising pharmacological agent for the treatment of pulmonary fibrosis.Reference: Acta Pharmacol Sin. 2018 Sep;39(9):1501-1512. https://pubmed.ncbi.nlm.nih.gov/29645000/ |
| Cell Assay | Immunofluorescent assay: Hypoxyprobe™-1 is a substituted 2-nitroimidazole named pimonidazole. It will bind to cells if the p O2 levels are less than 10 mmHg, so it is often used as a probe for detecting cell hypoxia. PLFs were cultured to 80% confluence. After 12 h of starvation, PLFs were incubated with 200 μmol/L hypoxyprobe and 10 μmol/L Neotuberostemonine (NTS) under normoxia, hypoxia (1% O2) or 100 μmol/L CoCl2 treatment at 37 °C for 12 h. PLFs were fixed and blocked with 3% bovine serum albumin (BSA), then treated with anti-hypoxyprobe and DAPI. The blank control was treated in the same manner. All the treated PLFs were examined under confocal scanning microscopy.Reference: Acta Pharmacol Sin. 2018 Sep;39(9):1501-1512. https://pubmed.ncbi.nlm.nih.gov/29645000/ |
| Animal Protocol | ICR male mice were randomly divided into 5 groups (n=20), ie, the sham, model, Neotuberostemonine (NTS), PND and Dig groups. After 12 h of fasting, mice were intratracheally injected with BLM (3.5 U/kg in 0.9% NaCl) or 0.9% NaCl (sham group) after anesthesia with 4% chloral hydrate (10 mL/kg). After 7 d of model formation, BLM-treated mice were orally administered Neotuberostemonine (NTS) (30 mg/kg), PDN (6.5 mg/kg) and an equivalent volume of the same menstruum (sham and model group) or intraperitoneally injected with Dig (1 mg/kg) once a day for 7 or 14 consecutive days. The lungs were excised on d 15 and 22 after BLM treatment.Reference: Acta Pharmacol Sin. 2018 Sep;39(9):1501-1512. https://pubmed.ncbi.nlm.nih.gov/29645000/ |
| References |
[1]. Neotuberostemonine attenuates bleomycin-induced pulmonary fibrosis by suppressing the recruitment and activation of macrophages. Int Immunopharmacol. 2016 Jul;36:158-164. |
| Additional Infomation |
Neotuberostemonine is an alkaloid. It has a role as a metabolite. Neotuberostemonine has been reported in Stemona japonica, Stemona phyllantha, and other organisms with data available. |
Solubility Data
| Solubility (In Vitro) |
DMSO : ~50 mg/mL (~133.16 mM) H2O : < 0.1 mg/mL |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6630 mL | 13.3152 mL | 26.6304 mL | |
| 5 mM | 0.5326 mL | 2.6630 mL | 5.3261 mL | |
| 10 mM | 0.2663 mL | 1.3315 mL | 2.6630 mL |