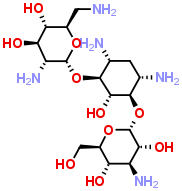
Physicochemical Properties
| Molecular Formula | C18H37N5O10 |
| Molecular Weight | 483.51 |
| Exact Mass | 483.254 |
| Elemental Analysis | C, 44.71; H, 7.71; N, 14.48; O, 33.09 |
| CAS # | 4696-76-8 |
| Related CAS # | 4696-76-8;29701-07-3 (sulfate); |
| PubChem CID | 439318 |
| Appearance | White to off-white solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 807.7±65.0 °C at 760 mmHg |
| Melting Point | 178-182° (dec) |
| Flash Point | 442.3±34.3 °C |
| Vapour Pressure | 0.0±6.5 mmHg at 25°C |
| Index of Refraction | 1.668 |
| LogP | -2.93 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 33 |
| Complexity | 639 |
| Defined Atom Stereocenter Count | 15 |
| SMILES | O([C@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])N([H])[H])O1)O[H])O[H])N([H])[H])[C@]1([H])[C@]([H])(C([H])([H])[C@]([H])([C@@]([H])([C@@]1([H])O[H])O[C@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])N([H])[H])O[H])N([H])[H])N([H])[H] |
| InChi Key | SKKLOUVUUNMCJE-FQSMHNGLSA-N |
| InChi Code | InChI=1S/C18H37N5O10/c19-2-6-11(26)12(27)9(23)17(30-6)32-15-4(20)1-5(21)16(14(15)29)33-18-13(28)8(22)10(25)7(3-24)31-18/h4-18,24-29H,1-3,19-23H2/t4-,5+,6+,7+,8-,9+,10+,11+,12+,13+,14-,15+,16-,17+,18+/m0/s1 |
| Chemical Name | (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-(((1R,2S,3S,4R,6S)-4,6-diamino-3-(((2S,3R,4S,5S,6R)-4-amino-3,5-dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-2-hydroxycyclohexyl)oxy)tetrahydro-2H-pyran-3,4-diol |
| Synonyms | Bekanamycin; NK-1006; NK1006; NK 1006; Nebramycin V |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Aminoglycoside |
| ln Vitro |
Bekanamycin, also known as Kanamycin B, is typically extracted from the broth of S. kanamyceticus and is a precursor for semisynthetic antibiotics like Arbekacin and Dibekacin[2]. While bekanamycin (also known as Kanamycin B) has no discernible effect on the configuration of the extracellularly recorded presynaptic action potential, it does, in a concentration-dependent manner, reduce the quantal content of the end-plate potentials reversibly. Bekanamycin causes evoked transmitter release to decrease, but this can be countered by raising the concentration of calcium outside the body or by using medications like aminopyridines, which significantly increase the release of transmitters from motor nerve terminals. Strong inhibitory effects of bekanamycin on transmitter release are likely caused by disruption of the calcium influx that takes place during motor nerve terminal depolarization[3]. |
| References |
[1]. Modulation of kanamycin B and kanamycin A biosynthesis in Streptomyces kanamyceticus viametabolic engineering. PLoS One. 2017 Jul 28;12(7):e0181971. [2]. Synthesis and Bioactivities of Kanamycin B-Derived Cationic Amphiphiles. J Med Chem. 2015 Dec 10;58(23):9124-32. [3]. Presynaptic effects of bekanamycin at the frog neuromuscular junction. Reversibility by calcium and aminopyridines. Eur J Pharmacol. 1981 Jul 10;72(4):271-80. |
| Additional Infomation |
Kanamycin B is a member of kanamycins. It is a conjugate base of a kanamycin B(5+). Bekanamycin has been reported in Apis cerana and Streptomyces kanamyceticus with data available. Bekanamycin is an aminoglycoside antibiotic derived from Streptomyces kanamyceticus, with antibacterial activity. Bekanamycin irreversibly binds to the bacterial 30S ribosomal subunit. Specifically, this antibiotic is lodged between 16S rRNA and S12 protein within the 30S subunit. This leads to interference with translational initiation complex, misreading of mRNA, thereby hampering protein synthesis and resulting in bactericidal effect. |
Solubility Data
| Solubility (In Vitro) |
H2O : ≥ 100 mg/mL (~206.82 mM) DMSO : ~1 mg/mL (~2.07 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: 25 mg/mL (51.71 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0682 mL | 10.3410 mL | 20.6821 mL | |
| 5 mM | 0.4136 mL | 2.0682 mL | 4.1364 mL | |
| 10 mM | 0.2068 mL | 1.0341 mL | 2.0682 mL |