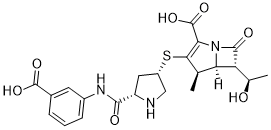
Ertapenem (L-749345; Invanoz; MK-0826; Invanz) is a 1-β-methyl carbapenem antibiotic marketed by Merck as Invanz. Ertapenem is a long-acting, broad-spectrum antibiotic of β-lactam subclass. Ertapenem has a broad spectrum of antibacterial activity including common aerobic and anaerobic bacteria and organisms with extended-spectrum β-lactamases. Ertapenem is an inhibitor of bacteria cell-wall synthesis, it acts by binding to penicillin binding proteins located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition of peptidoglycan synthesis results in weakening and lysis of the cell wall and cell death. Erapenem is resistant to hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases.
Physicochemical Properties
| Molecular Formula | C22H25N3O7S |
| Molecular Weight | 475.5 |
| Exact Mass | 475.141 |
| Elemental Analysis | C, 55.57; H, 5.30; N, 8.84; O, 23.55; S, 6.74 |
| CAS # | 153832-46-3 |
| Related CAS # | Ertapenem sodium;153773-82-1;Ertapenem disodium;153832-38-3 |
| PubChem CID | 150610 |
| Appearance | Solid powder |
| Density | 1.6±0.1 g/cm3 |
| Boiling Point | 813.9±65.0 °C at 760 mmHg |
| Melting Point | 230-234 |
| Flash Point | 446.0±34.3 °C |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.700 |
| LogP | -1.07 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 33 |
| Complexity | 893 |
| Defined Atom Stereocenter Count | 6 |
| SMILES | S([C@@H]1CN[C@H](C(NC2=CC=CC(C(=O)O)=C2)=O)C1)C1=C(C(=O)O)N2C([C@]([H])([C@@H](C)O)[C@@]2([H])[C@H]1C)=O |
| InChi Key | JUZNIMUFDBIJCM-ANEDZVCMSA-N |
| InChi Code | InChI=1S/C22H25N3O7S/c1-9-16-15(10(2)26)20(28)25(16)17(22(31)32)18(9)33-13-7-14(23-8-13)19(27)24-12-5-3-4-11(6-12)21(29)30/h3-6,9-10,13-16,23,26H,7-8H2,1-2H3,(H,24,27)(H,29,30)(H,31,32)/t9-,10-,13+,14+,15-,16-/m1/s1 |
| Chemical Name | (4R,5S,6S)-3-[(3S,5S)-5-[(3-Carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
| Synonyms | MK 826; L-749345; MK-826; L749345; MK826; L 749345; MK-0826; MK 0826; MK0826; Ertapenem Sodium; Trade Name: Invanoz; Invanz |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | β-lactam |
| ln Vitro | Ertapenem (0-100 μg/mL approximately, 48 hours) is effective against 99.1% of all anaerobes, with MICs for B.fragilis and B.vulgatus species of ≥8 μg/mL and a mode MIC of 0.12 μg/mL and MIC90 of 1 μg/mL, respectively[1]. |
| ln Vivo |
In a S. aureus thigh tissue infection model, ertapenem (subcutaneous injection, 0–10 mg/kg, 0-120 h after infection) reduces the organism by > 3 log10 CFU at 10 mg/kg and keeps the activity at 3.3 and 4.4 log10 CFU eliminated at 2 mg/kg[2]. In addition to being active against all gram-positive organisms, ertapenem (subcutaneous injection, 4 hours after infection, systemic infection model) is also active against gram-negative organisms with ED50s of less than 0.25 mg/kg/dose[2]. |
| Cell Assay |
Cell Line: B. fragilis (ATCC 25285), B. thetaiotaomicron (ATCC 29741), and Eubacterium lentum (ATCC 43055) Concentration: 0-100 μg/mL approximately Incubation Time: 48 h Result: 98.8% of the isolates in the B. fragilis group were susceptible, and 99.1% of all isolates were inhibited with a mode MIC of 0.12 μg/mL and MIC90 of 1 μg/mL. |
| Animal Protocol |
Animal Model: S. aureus thigh tissue infection model (DBA/2 mice)[2] Dosage: 0.5,1, 2, 5, 10 mg/kg (given at 2, 6, 10, 24, 48, 72, 96, 120 h) Administration: Subcutaneous injection (0.5 mL after infection) Result: showed a reduction in organism of > 3 log10 CFU at 10 mg/kg when compared to controls not treated with antibiotics. kept up the activity at 2 mg/kg, eliminating 3.3 and 4.4 log10 CFU. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Ertapenem is almost completely absorbed following intramuscular administration, with a mean bioavailability of approximately 90%. Plasma concentrations of ertapenem are similar whether given intramuscularly or intravenously; however, the peak concentrations are lower when given via the intramuscular route. The time to reach the Cmax (Tmax) is slightly longer when given via the intramuscular route. Following daily intramuscular administration of one gram of ertapenem, the Tmax was approximately 2.3 hours. In healthy young adults who received a single 30-minute intravenous infusion of one gram of ertapenem, the Cmax was 155 µG/mL at 0.5 hours postdose. Ertapenem predominantly undergoes renal elimination, where it undergoes glomerular filtration and net tubular secretion. In healthy young adults who received one gram of IV radiolabeled ertapenem, approximately 80% of the radioactivity was recovered in urine and 10% of the radioactivity was recovered in feces. The mean percentage of the administered dose excreted in urine was 17.4% during 0-2 hours postdose, 5.4% during 4-6 hours postdose, and 2.4% during 12-24 hours postdose. Of the 80% radioactivity in urine, about 38% accounted for unchanged ertapenem and 37% accounted for its ring-opened metabolite. The apparent volume of distribution at steady state (Vss) of ertapenem is approximately 0.12 L/kg in adults, 0.2 L/kg in children three months to 12 years of age, and 0.16 L/kg in adolescents 13 to 17 years of age. Ertapenem does not accumulate. The mean plasma clearance in healthy young adults was approximately 1.8 L/hour. The mean renal clearance of intact ertapenem was 12.8 mL/min compared with a total clearance of 28.4 mL/min. Ertapenem, reconstituted with 1% lidocaine HCl injection, USP (in saline without epinephrine), is almost completely absorbed following intramuscular (IM) administration at the recommended dose of 1 g. The mean bioavailability is approximately 90%. Following 1 g daily IM administration, mean peak plasma concentrations (Cmax) are achieved in approximately 2.3 hours (Tmax). Ertapenem is highly bound to human plasma proteins, primarily albumin. In healthy young adults, the protein binding of ertapenem decreases as plasma concentrations increase, from approximately 95% bound at an approximate plasma concentration of <100 micrograms (ug)/mL to approximately 85% bound at an approximate plasma concentration of 300 ug/mL. The apparent volume of distribution at steady state (Vss) of ertapenem in adults is approximately 0.12 liter/kg, approximately 0.2 liter/kg in pediatric patients 3 months to 12 years of age and approximately 0.16 liter/kg in pediatric patients 13 to 17 years of age. The concentration of ertapenem in breast milk from 5 lactating women with pelvic infections (5 to 14 days postpartum) was measured at random time points daily for 5 consecutive days following the last 1 g dose of intravenous therapy (3-10 days of therapy). The concentration of ertapenem in breast milk within 24 hours of the last dose of therapy in all 5 women ranged from <0.13 (lower limit of quantitation) to 0.38 ug/mL; peak concentrations were not assessed. By day 5 after discontinuation of therapy, the level of ertapenem was undetectable in the breast milk of 4 women and below the lower limit of quantitation (<0.13 ug/mL) in 1 woman. For more Absorption, Distribution and Excretion (Complete) data for Ertapenem (18 total), please visit the HSDB record page. Metabolism / Metabolites In healthy young adults, unchanged ertapenem accounted for most plasma radioactivity. The major metabolite of ertapenem is the ring-opened derivative formed by dehydropeptidase I-mediated hydrolysis of the beta-lactam ring. This metabolite is pharmacologically inactive. Dehydropeptidase I (DHP-I) is found predominantly in the kidneys. Hepatic metabolism is negligible. The disposition and metabolism of ertapenem, a carbapenem antibiotic, was examined in rat, monkey and man. Sprague-Dawley rats and Rhesus monkeys were given, by intravenous administration, radiolabelled doses of ertapenem (60 and 30 mg kg(-1), respectively), and healthy normal volunteers received a single fixed dose of 1000 mg. Urine and feces were collected for determination of total radioactivity. In healthy volunteers, (14)C-ertapenem was eliminated by a combination of hydrolytic metabolism to a beta-lactam ring-opened derivative and renal excretion of unchanged drug. Approximately equal amounts were excreted as a beta-lactam ring-opened metabolite and unchanged drug (36.7 and 37.5% of dose, respectively). A secondary amide hydrolysis product accounted for about 1% of the dose in man. About 10% of the administered radioactivity was recovered in feces, which suggested that a minor fraction underwent biliary and/or intestinal excretion. In animals, a greater fraction of the dose was eliminated via metabolism; excretion of unchanged drug accounted for 17 and 5% of dose in rats and monkeys, respectively. In monkeys, the beta-lactam ring-opened and amide hydrolysis metabolites accounted for 74.8 and 7.59% of the dose, respectively, whereas in rats, these metabolites accounted for 31.9 and 20% of dose, respectively. In vitro studies with fresh rat tissue homogenates indicated that lung and kidney were the primary organs involved in mediating formation of the beta-lactam ring-opened metabolite. The specific inhibitor of dehydropeptidase-I, cilastatin, inhibited the in vivo and in vitro metabolism of ertapenem in rats, which suggested strongly that the hydrolysis of ertapenem in lung and kidney was mediated by this enzyme. Ertapenem is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases. Ertapenem is hydrolyzed by metallo-beta-lactamases. In healthy young adults, after infusion of 1 g IV radiolabeled ertapenem, the plasma radioactivity consists predominantly (94%) of ertapenem. The major metabolite of ertapenem is the inactive ring-opened derivative formed by hydrolysis of the beta-lactam ring. Biological Half-Life The mean plasma half-life was approximately four hours in healthy young adults and adolescents and approximately 2.5 hours in children three to 12 years of age. The long half-life of ertapenem can be explained by its high protein binding. The drug has a mean plasma half-life of approximately 4 hours and may be administered once daily. The mean plasma half-life in pediatric patients 13 to 17 years of age is approximately 4 hours and approximately 2.5 hours in pediatric patients 3 months to 12 years of age. The mean plasma t(1/2) ranged from 3.8 to 4.4 hr. |
| Toxicity/Toxicokinetics |
Hepatotoxicity Mild, transient, asymptomatic elevations in serum aminotransferase levels occur in about 5% of patients receiving parenteral ertapenem for 5 to 14 days. These abnormalities are usually self-limited and asymptomatic. In the limited period that it has been available, no cases of hepatitis with jaundice have been reported. Nevertheless, several instances of cholestatic jaundice arising during or shortly after therapy have been reported with other carbapenems. The latency to onset has been within 1 to 3 weeks and the pattern of enzyme elevations is usually cholestatic. Immunoallergic features can occur but autoantibodies are rare. The course is usually self-limiting, but at least one case of vanishing bile duct syndrome related to a carbapenem has been reported. Ertapenem and other carbapenems have not been linked to cases of acute liver failure. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that ertapenem produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush has been reported with beta-lactams, but these effects have not been adequately evaluated. Ertapenem is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ertapenem binds to plasma proteins in a concentration-dependent manner. It is highly bound to human plasma proteins, primarily to albumin. Protein binding is saturable at higher doses, at which the unbound fraction of the drug increases disproportionately. In healthy young adults, the protein binding of ertapenem decreased as drug plasma concentrations increased. At an approximate plasma concentration of <100 micrograms (mcg)/mL, ertapenem was 95% bound, and this percentage dropped to 85% when the plasma concentration increased to 300 mcg/mL. |
| References |
[1]. Ertapenem (MK-0826), a new carbapenem: comparative in vitro activity against clinically significant anaerobes. Diagn Microbiol Infect Dis. 2002 Oct;44(2):181-6. [2]. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749,345). Antimicrob Agents Chemother. 1998 Aug;42(8):1996-2001. [3]. Antimicrob Agents Chemother.2011 Nov;55(11):4943-60. |
| Additional Infomation |
Ertapenem is meropenem in which the one of the two methyl groups attached to the amide nitrogen is replaced by hydrogen while the other is replaced by a 3-carboxyphenyl group. The sodium salt is used for the treatment of moderate to severe susceptible infections including intra-abdominal and acute gynaecological infections, pneumonia, and infections of the skin and of the urinary tract. It has a role as an antibacterial drug. It is a carbapenemcarboxylic acid and a pyrrolidinecarboxamide. It is a conjugate acid of an ertapenem(1-). Ertapenem is a 1-β methyl-carbapenem that is structurally related to beta-lactam antibiotics. It was first authorized for use in the US in November 2001 and in Europe in April 2002. Shown to be effective against a wide range of Gram-positive and Gram-negative aerobic and anaerobic bacteria, ertapenem is used to treat various bacterial infections. Ertapenem is a Penem Antibacterial. Ertapenem is a broad spectrum carbapenem antibiotic used primarily for the treatment of aerobic gram-negative bacterial infections. Ertapenem, like other carbapenems, is associated with transient and asymptomatic elevations in serum enzymes. The carbapenems have also been linked to rare instances of clinically apparent, acute cholestatic liver injury. Ertapenem is a 1-beta-methyl carbapenem and broad-spectrum beta-lactam antibiotic with bactericidal property. Ertapenem binds to penicillin binding proteins (PBPs) located on the bacterial cell wall, in particular PBPs 2 and 3, thereby inhibiting the final transpeptidation step in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Inhibition results in a weakening and subsequent lysis of the cell wall leading to cell death of Gram-positive and Gram-negative aerobic and anaerobic pathogens. This agent is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, cephalosporinases and extended-spectrum beta-lactamases. A carbapenem derivative antibacterial agent that is more stable to renal dehydropeptidase I than IMIPENEM, but does not need to be given with an enzyme inhibitor such as CILASTATIN. It is used in the treatment of Gram-positive and Gram-negative bacterial infections including intra-abdominal infections, acute gynecological infections, complicated urinary tract infections, skin infections, and respiratory tract infections. It is also used to prevent infection in colorectal surgery. See also: Ertapenem Sodium (has salt form). Drug Indication Ertapenem is indicated to treat the following moderate to severe infections caused by susceptible bacteria in adult and pediatric patients (three months of age and older): - Complicated intra-abdominal infections. - Complicated skin and skin structure infections, including diabetic foot infections without osteomyelitis. - Community-acquired pneumonia. - Complicated urinary tract infections, including pyelonephritis. - Acute pelvic infections, including postpartum endomyometritis, septic abortion and post-surgical gynecologic infections. - Acute gynecological infections. Ertapenem is also used in adults for the prophylaxis of surgical site infection following elective colorectal surgery. TreatmentErtapenem SUN is indicated in paediatric patients (3 months to 17 years of age) and in adults for the treatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required (see sections 4. 4 and 5. 1): - Intra-abdominal infections- Community acquired pneumonia- Acute gynaecological infections- Diabetic foot infections of the skin and soft tissue (see section 4. 4)PreventionErtapenem SUN is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery (see section 4. 4). Consideration should be given to official guidance on the appropriate use of antibacterial agents. TreatmentTreatment of the following infections when caused by bacteria known or very likely to be susceptible to ertapenem and when parenteral therapy is required: intra-abdominal infections; community-acquired pneumonia; acute gynaecological infections; diabetic foot infections of the skin and soft tissue. PreventionInvanz is indicated in adults for the prophylaxis of surgical site infection following elective colorectal surgery. Consideration should be given to official guidance on the appropriate use of antibacterial agents. Mechanism of Action Ertapenem exhibits a bactericidal mode of action. It works by binding to and inhibiting bacterial penicillin-binding proteins (PBPs). In _Escherichia coli_, it has a strong affinity toward PBPs 1a, 1b, 2, 3, 4 and 5 with preferential binding to PBPs 2 and 3. Upon binding to PBPs, ertapenem inhibits bacterial cell wall synthesis by interfering with the lengthening and strengthening of the peptidoglycan portion of the cell wall, thereby inhibiting cell wall synthesis. Ertapenem is a synthetic carbapenem beta-lactam antibiotic that is structurally and pharmacologically related to imipenem and meropenem. Like meropenem but unlike imipenem, ertapenem has a methyl group at position 1 of the 5-membered ring, which confers stability against hydrolysis by dehydropeptidase 1 (DHP 1) present on the brush border of proximal renal tubular cells, and therefore does not require concomitant administration with a DHP-1 inhibitor such as cilastatin. Ertapenem has in vitro activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria. The bactericidal activity of ertapenem results from the inhibition of cell wall synthesis and is mediated through ertapenem binding to penicillin binding proteins (PBPs). In Escherichia coli, it has strong affinity toward PBPs 1a, 1b, 2, 3, 4 and 5 with preference for PBPs 2 and 3. Antimicrobials are the most frequently implicated class of drugs in drug-induced seizure, with beta-lactams being the class of antimicrobials most often implicated. The seizure-inducing potential of the carbapenem subclass may be directly related to their beta-lactam ring structure. Data on individual carbapenems and seizure activity are scarce. To evaluate the available evidence on the association between carbapenem agents and seizure activity, /investigators/ conducted a literature search of the MEDLINE (1966-May 2010), EMBASE (1974-May 2010), and International Pharmaceutical Abstracts (1970-May 2010) databases. Reference citations from the retrieved articles were also reviewed. Mechanistically, seizure propensity of the beta-lactams is related to their binding to gamma-aminobutyric acid (GABA) receptors. There are numerous reports of seizure activity associated with imipenem-cilastatin, with seizure rates ranging from 3-33%. For meropenem, doripenem, and ertapenem, the seizure rate for each agent is reported as less than 1%. However, as their use increases and expands into new patient populations, the rate of seizures with these agents may increase. High-dose therapy, especially in patients with renal dysfunction, preexisting central nervous system abnormalities, or a seizure history increases the likelihood of seizure activity. ... |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1030 mL | 10.5152 mL | 21.0305 mL | |
| 5 mM | 0.4206 mL | 2.1030 mL | 4.2061 mL | |
| 10 mM | 0.2103 mL | 1.0515 mL | 2.1030 mL |