Physicochemical Properties
| Molecular Formula | C7H6N2 |
| Molecular Weight | 118.14 |
| Exact Mass | 118.053 |
| CAS # | 271-44-3 |
| PubChem CID | 9221 |
| Appearance | Light brown to yellow solid powder |
| Density | 1.2±0.1 g/cm3 |
| Boiling Point | 270.0±9.0 °C at 760 mmHg |
| Melting Point | 145-148 °C(lit.) |
| Flash Point | 128.5±11.7 °C |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.697 |
| LogP | 1.82 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Heavy Atom Count | 9 |
| Complexity | 103 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | BAXOFTOLAUCFNW-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C7H6N2/c1-2-4-7-6(3-1)5-8-9-7/h1-5H,(H,8,9) |
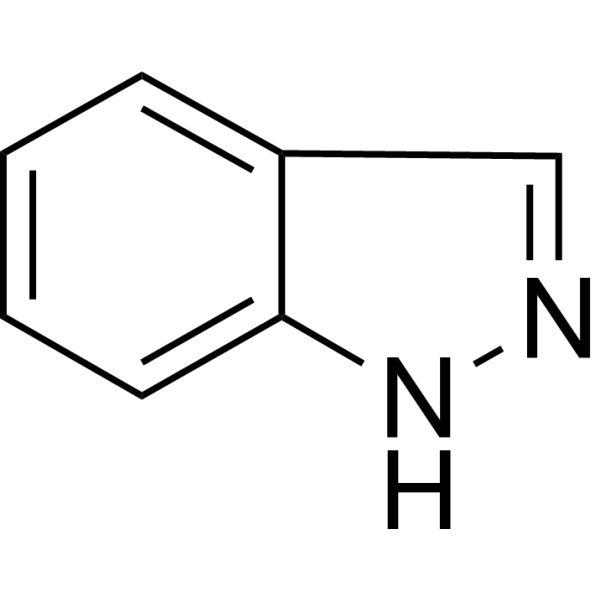
| Chemical Name | 1H-indazole |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Axitinib (HY-10065), Lonidamine (HY-B0486), and Pazopanib (HY-10208) are examples of indazole-based agents that have been used in cancer research and show promise as useful building blocks for the creation of new anticancer drugs. In addition, indazole compounds themselves may have anticancer properties[1]. Because indazoles inhibit kinase and monoamine oxidase (MAO) enzymes such as leucinerich repeat kinase enzyme 2 (LRRK2) and glycogen synthase kinase 3 (GSK3), they have demonstrated strong effects against neurological disorders[2]. Several natural and synthetic indazole derivatives, including as SCH772984 (HY-50846), Zanubrutinib (HY-101474A), Nigellicine, Nigellamine, and Nigellidine, have demonstrated notable efficacy in treating a range of gastrointestinal disorders[4]. |
| References |
[1]. The Anticancer Activity of Indazole Compounds: A Mini Review. Curr Top Med Chem. 2021;21(5):363-376. [2]. Importance of Indazole against Neurological Disorders. Curr Top Med Chem. 2022;22(14):1136-1151. [3]. Indazole and its Derivatives in Cardiovascular Diseases: Overview, Current Scenario, and Future Perspectives. Curr Top Med Chem. 2022;22(14):1177-1188. [4]. Indazole Derivatives Effective against Gastrointestinal Diseases. Curr Top Med Chem. 2022;22(14):1189-1214. [5]. Indazole as a Privileged Scaffold: The Derivatives and their Therapeutic Applications. Anticancer Agents Med Chem. 2021;21(7):839-860. |
| Additional Infomation |
1H-indazole is an indazole. It is a tautomer of a 2H-indazole. A group of heterocyclic aromatic organic compounds consisting of the fusion of BENZENE and PYRAZOLES. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 8.4645 mL | 42.3227 mL | 84.6453 mL | |
| 5 mM | 1.6929 mL | 8.4645 mL | 16.9291 mL | |
| 10 mM | 0.8465 mL | 4.2323 mL | 8.4645 mL |