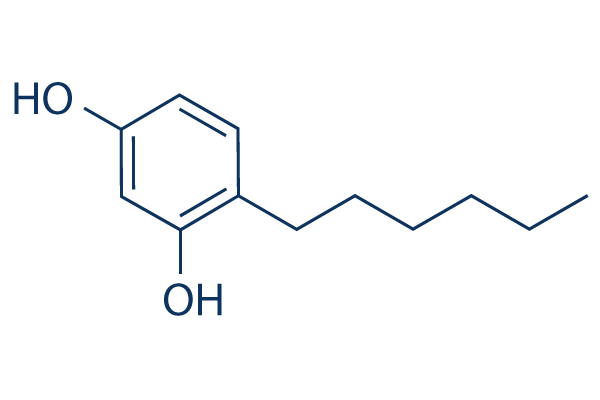
Hexylresorcinol (4-Hexylresorcinol) is an organic compound with local anaesthetic, antiseptic and anthelmintic properties, is a potent inhibitor of mushroom tyrosinase. The IC50 values of hexylresorcinol for monophenolase is 1.24 μM and for diphenolase is 0.85 μM. Studies showed hexylresorcinol could inhibit both mono- and di-phenolase activity of mushroom tyrosinase. Moreover, hexylresorcinol at 2 μM lengthened the lag period from 98 s to 26. Hexylresorcinol could also display reversible inhibition of the enzyme.
Physicochemical Properties
| Molecular Formula | C12H18O2 | |
| Molecular Weight | 194.27 | |
| Exact Mass | 194.13 | |
| Elemental Analysis | C, 74.19; H, 9.34; O, 16.47 | |
| CAS # | 136-77-6 | |
| Related CAS # |
|
|
| PubChem CID | 3610 | |
| Appearance |
Pale yellow, heavy liq becoming solid on standing at room temp; needles from benzene or petroleum ether WHITE, OR YELLOWISH WHITE, NEEDLE-SHAPED CRYSTALS; ACQUIRES BROWNISH PINK TINT ON EXPOSURE TO LIGHT & AIR |
|
| Density | 1.0±0.1 g/cm3 | |
| Boiling Point | 329.5±12.0 °C at 760 mmHg | |
| Melting Point | 65-67 °C(lit.) | |
| Flash Point | 155.2±14.2 °C | |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C | |
| Index of Refraction | 1.540 | |
| LogP | 3.88 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 14 | |
| Complexity | 147 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O([H])C1C([H])=C(C([H])=C([H])C=1C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])O[H] |
|
| InChi Key | WFJIVOKAWHGMBH-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C12H18O2/c1-2-3-4-5-6-10-7-8-11(13)9-12(10)14/h7-9,13-14H,2-6H2,1H3 | |
| Chemical Name | 4-Hexylresorcinol | |
| Synonyms | Hexylresorcinol; Ascaricid; Ascarinol; Ascaryl; NSC 1570; NSC-1570; NSC1570 | |
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Owing to the poor absorption of hexylresorcinol, systemic exposure and symptoms are unusual. When two men received doses of 1 g of hexylresorcinol, an average of 18% of the dose was recovered in the urine within the first 12 hours - thereafter, the compound was not detected in urine samples. Readily accessible data regarding the volume of distribution of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area. Readily accessible data regarding the clearance of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area. Dogs were given single doses of 1 or 3 g 4-hexylresorcinol (equivalent to 100 or 300 mg/kg bw) as crystals in gelatin capsules or as a solution in olive oil, and excretion monitored in urine and feces. After administration of 1 g crystalline compound, 29% of the dose was detected in urine and 67% in feces; when the dose was increased to 3 g, 17% was excreted in urine and 73% in feces. Urinary excretion was rapid, mainly in the first 6 hr, and levels were virtually undetectable 12 hr after the lower dose and 24-36 hr following the higher dose. When 4-hexylresorcinol was administered in olive oil, a dose of 1 g resulted in 17% being excreted in urine and 76% in feces, while 10% was excreted in urine and 80% in feces following a dose of 3 g. When two men received doses of 1 g 4-hexylresorcinol, an average of 18% of the dose was recovered in urine within the first 12 hr; thereafter the compound was not detected in urine samples. Fecal excretion accounted for 64% of the dose. Metabolism / Metabolites Regarding the metabolism of hexylresorcinol, it has been reported that excretion of the compound in the urine is largely in the form of an ethereal sulfate conjugate. Human metabolite of 4-hexylresorcinol is ethereal sulfate. /From table/ It has been reported that 4-hexylresorcinol is excreted via the urine mainly in the form of an ethereal sulfate conjugate ... Biological Half-Life Readily accessible data regarding the half-life of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area. |
||
| Toxicity/Toxicokinetics |
Protein Binding Readily accessible data regarding the protein binding of hexylresorcinol is not available. Nevertheless, when hexylresorcinol is employed in its primary indication as a topical antiseptic or an oral anesthetic, it is generally accepted that pharmacokinetic considerations do not arise since the pharmacological action is local to the topically applied or oro-pharyngeal cavity area. Non-Human Toxicity Values LD50 Rat oral 550 mg/kg LD50 Mouse sc 750-1000 mg/kg bw (5% in olive oil) LD50 Mouse ip 200 mg/kg bw (5% in olive oil) LD50 Mouse ip 300 mg/kg bw (1% aqueous emulsion) For more Non-Human Toxicity Values (Complete) data for HEXYLRESORCINOL (7 total), please visit the HSDB record page. |
||
| References | Protein J.2004 Feb;23(2):135-41;Genetika.2005Aug;41(8):1045-8. | ||
| Additional Infomation |
Therapeutic Uses Anti-Infective Agents, Local; Antinematodal Agents; Antiplatyhelmintic Agents It is commonly employed in 1:1000 soln or glycerite in mouthwashes or pharyngeal antiseptic preparation. MEDICATION (VET): Rare now, as anthelmintic especially since introduction of dichlorvos and other drugs. Topically it is effective bacteriostatic, bactericidal, virucidal, fungistatic, and fungicidal agent at dilutions greater than 1:1000 (0.1%), although latter is safe topically. In ringworm therapy with Aminoacridinium = Aacrisorcin ... MEDICATION (VET): Effective against many viruses when aerosoled at 5 mg/cu m. ... Administer orally in oil to reduce local irritation. For more Therapeutic Uses (Complete) data for HEXYLRESORCINOL (7 total), please visit the HSDB record page. Drug Warnings Hexylresorcinol should not be dispensed in ordinary, hard-gelatin capsules as these quickly become brittle, and may break in mouth causing caustic burns. Hexylresorcinol, given orally, is ineffective but when given by enema, tedious and unpleasant experience for patients, there is immediate symptomatic relief, although cures are rarely attained. Care should be taken that pills containing drug are swallowed whole or painful ulceration of oral mucous membrane may result. Pharmacodynamics Hexylresorcinol is a phenol derivative, and in typical therapeutic usage is primarily a local anesthetic for topical use on the mucous membranes of the mouth and throat. The local anesthetic like properties of hexylresorcinol is likely due to its sodium channel blocking effects. The agent also demonstrates mild antiseptic activity as well as an apparent anti-inflammatory, demulcent action. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 38~250 mg/mL (195.6 ~1286.87 mM ) Ethanol : ~100 mg/mL (~514.75 mM ) H2O : ~1 mg/mL (~5.15 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (12.87 mM) (saturation unknown) in 10% EtOH + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (12.87 mM) (saturation unknown) in 10% EtOH + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (12.87 mM) (saturation unknown) in 10% EtOH + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear EtOH stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: ≥ 2.08 mg/mL (10.71 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: ≥ 2.08 mg/mL (10.71 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 6: ≥ 2.08 mg/mL (10.71 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 7: 10% EtOH+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (12.87 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.1475 mL | 25.7374 mL | 51.4748 mL | |
| 5 mM | 1.0295 mL | 5.1475 mL | 10.2950 mL | |
| 10 mM | 0.5147 mL | 2.5737 mL | 5.1475 mL |