Physicochemical Properties
| Molecular Formula | C29H35CLN2O4 |
| Molecular Weight | 511.06 |
| Exact Mass | 510.2285 |
| Elemental Analysis | C, 68.16; H, 6.90; Cl, 6.94; N, 5.48; O, 12.52 |
| CAS # | 1808119-16-5 |
| PubChem CID | 156484476 |
| Appearance | White to off-white solid powder |
| LogP | 3.8 |
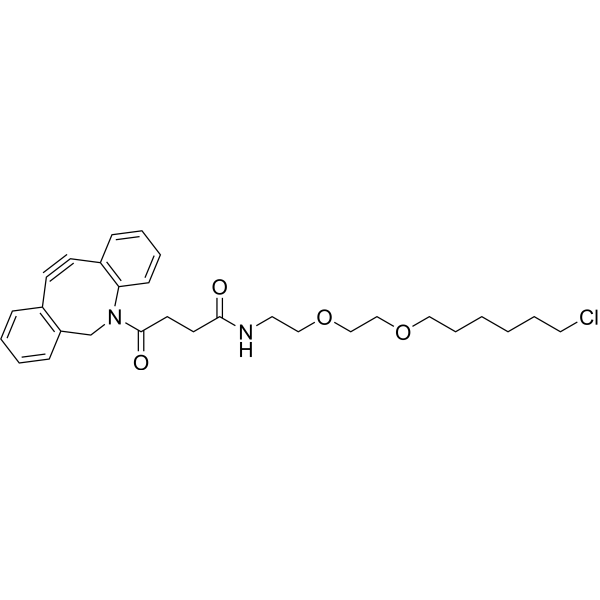
| SMILES | O=C(NCCOCCOCCCCCCCl)CCC(N1C2=CC=CC=C2C#CC3=CC=CC=C3C1)=O |
| InChi Key | IPBMADSLMZUFQN-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C29H35ClN2O4/c30-17-7-1-2-8-19-35-21-22-36-20-18-31-28(33)15-16-29(34)32-23-26-11-4-3-9-24(26)13-14-25-10-5-6-12-27(25)32/h3-6,9-12H,1-2,7-8,15-23H2,(H,31,33) |
| Chemical Name | 4-(2-Azatricyclo[10.4.0.04,9]hexadeca-1(16),4,6,8,12,14-hexaen-10-yn-2-yl)-N-[2-[2-(6-chlorohexoxy)ethoxy]ethyl]-4-oxobutanamide |
| Synonyms | Halo-DBCO; 1808119-16-5; 4-(2-azatricyclo[10.4.0.04,9]hexadeca-1(16),4,6,8,12,14-hexaen-10-yn-2-yl)-N-[2-[2-(6-chlorohexoxy)ethoxy]ethyl]-4-oxobutanamide; HaloTag DBCO Ligand; SCHEMBL23511160; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Click chemistry reagent |
| ln Vitro | Bioorthogonal reactions, including the strain-promoted azide-alkyne cycloaddition (SPAAC) and inverse electron demand Diels-Alder (iEDDA) reactions, have become increasingly popular for live-cell imaging applications. However, the stability and reactivity of reagents has never been systematically explored in the context of a living cell. Here we report a universal, organelle-targetable system based on HaloTag protein technology for directly comparing bioorthogonal reagent reactivity, specificity, and stability using clickable HaloTag ligands in various subcellular compartments. This system enabled a detailed comparison of the bioorthogonal reactions in live cells and informed the selection of optimal reagents and conditions for live-cell imaging studies. We found that the reaction of sTCO with monosubstituted tetrazines is the fastest reaction in cells; however, both reagents have stability issues. To address this, we introduced a new variant of sTCO, Ag-sTCO, which has much improved stability and can be used directly in cells for rapid bioorthogonal reactions with tetrazines. Utilization of Ag complexes of conformationally strained trans-cyclooctenes should greatly expand their usefulness especially when paired with less reactive, more stable tetrazines.[1] |
| Cell Assay |
Labeling of HaloTag Fusion Proteins with Bioorthogonal Ligands and Evaluation of SPAAC and iEDDA Reactions in Live Cells[1] HeLa cells expressing HaloTag constructs were treated in 6-well dishes with 1 mL of 10 μM HaloTag ligands 1–12 in growth media for 0.5 h at 37 °C/5% CO2. Samples labeled with 12 served as a positive control to determine the maximum amount of HaloTag protein labeling per experiment. Cells were washed three times in DPBS and incubated in 2 mL new media for 1 h with one media change to remove unbound HaloTag ligands. For SPAAC reactions, HeLa cells were labeled with chloroalkane ligands 1–3 followed by either a dose response of 50 nM to 250 μM fluorophores 15–17 for 2 h, or a timecourse of 25 μM fluorophore for 30 s to 4 h in growth media. Reactions were immediately quenched by washing cells two times in 500 μM azide-amine 24, DBCO-amine 26 (Click Chemistry Tools), or BCN-amine 25 in PBS (SPAAC Quench buffer). For iEDDA, HeLa cells labeled with chloroalkane ligands 2, and 4–11 were treated with either a dose response from 1 nM-20 μM fluorophores 13, 14, 17, and 18 for 1 h, or a timecourse of 2 μM fluorophore for 10 s-2 h in growth media. Cells were quenched by washing two times in 100 μM Tz-amine 27 (Click Chemistry Tools) or TCO-amine 28 in PBS (iEDDA Quench buffer). Cells were scraped in 1 mL quench buffer, spun at 2000g for 3 min, the buffer was aspirated and cell pellets were immediately frozen on dry ice. |
| References |
[1]. Systematic Evaluation of Bioorthogonal Reactions in Live Cells with Clickable HaloTag Ligands: Implications for Intracellular Imaging. J Am Chem Soc. 2015 Sep 9;137(35):11461-75. |
| Additional Infomation | Overall, the HaloTag model system provides a unique and unbiased method to systematically evaluate bioorthogonal labeling strategies directly inside living mammalian cells, as well as in different subcellular organelles. Using this approach, researchers were able to rapidly assess various bioorthogonal groups for fast, efficient, and selective SPAAC and iEDDA ligations for live-cell imaging applications. While the SPAAC reactions were slower overall, researchers were still able to identify conditions for intracellular live-cell imaging studies using TAMRA fluorophores for both BCN and DBCO. However, in many cases the high fluorophore concentrations required for live-cell labeling studies limits the fluorophore selection due to background fluorescence accumulation. Unexpectedly, researchers discovered that the SPAAC reaction of DBCO 1 when conjugated to HaloTag proteins was nearly as rapid as the iEDDA reaction with slower dienophiles, TCO and BCN, in different subcellular organelles of live cells. This rate acceleration allowed the use of lower concentrations of the fluorophore-azide reporter and suggests that this SPAAC reaction may have utility for intracellular imaging applications where DBCO is used as a tag. When DBCO was linked to the reporter, researchers observed extensive background labeling at the high concentrations required for the SPAAC reaction, limiting its use in these instances.[1] |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9567 mL | 9.7836 mL | 19.5672 mL | |
| 5 mM | 0.3913 mL | 1.9567 mL | 3.9134 mL | |
| 10 mM | 0.1957 mL | 0.9784 mL | 1.9567 mL |