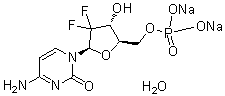
Gemcitabine monophosphate disodium (GemMP) is a monophosphate analog of Gemcitabine (formerly also known as LY-188011, NSC 613327; dFdC; dFdCyd; gemcitabine; trade name: Gemzar), an antimetabolite anticancer drug, which is a potent DNA synthesis inhibitor with IC50 of 50 nM, 40 nM, 18 nM and 12 nM in PANC1, MIAPaCa2, BxPC3 and Capan2 cells, respectively. The active metabolites difluorodeoxycytidine di- and triphosphate (dFdCDP, dFdCTP) are produced intracellularly during the conversion of gemcitabine. As ribonucleotide reductase is inhibited by dFdCDP, there is less deoxynucleotide available for DNA synthesis.
Physicochemical Properties
| Molecular Formula | C9H12F2N3NA2O8P |
| Molecular Weight | 405.156991958618 |
| Exact Mass | 387.001 |
| Elemental Analysis | C, 26.68; H, 2.99; F, 9.38; N, 10.37; Na, 11.35; O, 31.59; P, 7.64 |
| CAS # | 1638288-31-9 |
| Related CAS # | 116371-67-6 (free acid);1638288-31-9 (disodium);95058-81-4;122111-03-9;210829-30-4 |
| PubChem CID | 135391057 |
| Appearance | White to off-white solid powder |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Heavy Atom Count | 24 |
| Complexity | 566 |
| Defined Atom Stereocenter Count | 3 |
| SMILES | C1=CN(C(=O)N=C1N)[C@H]2C([C@@H]([C@H](O2)COP(=O)([O-])[O-])O)(F)F.[Na+].[Na+] |
| InChi Key | JVSFEPHISQCATF-AGPLKARSSA-L |
| InChi Code | InChI=1S/C9H12F2N3O7P.2Na/c10-9(11)6(15)4(3-20-22(17,18)19)21-7(9)14-2-1-5(12)13-8(14)16;;/h1-2,4,6-7,15H,3H2,(H2,12,13,16)(H2,17,18,19);;/q;2*+1/p-2/t4-,6-,7-;;/m1../s1 |
| Chemical Name | disodium;[(2R,3R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-4,4-difluoro-3-hydroxyoxolan-2-yl]methyl phosphate |
| Synonyms | GemMP; Gemcitabine monophosphate disodium; Gemcitabine monophosphate disodium salt monohydrate; Gemcitabine monophosphate disodium salt; OO7O3T57O3; Gemcitabine-5'-monophosphate disodium; disodium;[(2R,3R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-4,4-difluoro-3-hydroxyoxolan-2-yl]methyl phosphate; Gemcitabine-5'-monophosphate disodium salt; 5'-Cytidylic acid, 2'-deoxy-2',2'-difluoro-, sodium salt (1:2); Sodium ((2R,3R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl)methyl phosphate; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | DNA synthesis; active intermediate of Gemcitabine |
| ln Vitro |
There is an urgent need for new therapeutics for the treatment of aggressive and metastatic refractory human non-small-cell lung cancer (NSCLC). Antiangiogenesis therapy and chemotherapy are the two major treatment options. Unfortunately, both types of therapies when used individually have their disadvantages. Integrating antiangiogenesis therapy with chemotherapy is expected to target the tumor's vascular endothelial cells and the tumor cells simultaneously. In this study, we coformulated Vascular endothelial growth factor (VEGF) siRNA targeting VEGFs and gemcitabine monophosphate (GMP) into a single cell-specific, targeted lipid/calcium/phosphate (LCP) nanoparticle formulation.[1] Combination chemotherapy is a common practice in clinical management of malignancy. Synergistic therapeutic outcome is only achieved when tumor cells are exposed to cells in an optimal ratio. However, due to diverse physicochemical properties of drugs, no free drug cocktails or nanomaterials are capable of co-loading and co-delivering drugs at an optimal ratio. Herein, we develop a novel nano-platform with precise ratiometric co-loading and co-delivery of two hydrophilic drugs for synergistic anti-tumor effects. Based on previous work, we utilize a solvent displacement method to ratiometrically load dioleoyl phosphatidic acid (DOPA)-gemcitabine monophosphate and DOPA coated cisplatin-precipitate nanocores into the same PLGA NP. These cores are designed to have similar hydrophobic surface properties. GMP and cisplatin are engineered into PLGA NP at an optimal synergistic ratio (5:1, mol:mol) with over 70% encapsulation efficiency and were ratiometrically taken up by tumor cells in vitro and in vivo[2]. |
| ln Vivo |
Antitumor effect of the combination therapy using LCP loaded with both VEGF siRNA and GMP was evaluated in both subcutaneous and orthotopic xenograft models of NSCLC with systemic administration. The improved therapeutic response, as compared with either VEGF siRNA or GMP therapy alone, was supported by the observation of 30-40% induction of tumor cell apoptosis, eightfold reduction of tumor cell proliferation and significant decrease of tumor microvessel density (MVD). The combination therapy led to dramatic inhibition of tumor growth, with little in vivo toxicity. In addition, the current studies demonstrated the possibility of incorporating multiple nucleic acid molecules and phosphorylated small-molecule drugs, targeting to different pathways, into a single nanoparticle formulation for profound therapeutic effect.[1] These PLGA NP exhibit synergistic anti-cancer effects in a stroma-rich bladder tumor model. A single injection of dual drugs in PLGA NP can significantly inhibit tumor growth. This nanomaterial-system solves problems related to ratiometric co-loading and co-delivery of different hydrophilic moieties and provides possibilities for co-loading hydrophilic drugs with hydrophobic drugs for combination therapy[2]. |
| References |
[1]. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther. 2013 Aug;21(8):1559-69. [2]. Nanoparticles with Precise Ratiometric Co-Loading and Co-Delivery of Gemcitabine Monophosphate and Cisplatin for Treatment of Bladder Cancer. Adv Funct Mater. 2014 Nov 12;24(42):6601-6611. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4682 mL | 12.3408 mL | 24.6816 mL | |
| 5 mM | 0.4936 mL | 2.4682 mL | 4.9363 mL | |
| 10 mM | 0.2468 mL | 1.2341 mL | 2.4682 mL |