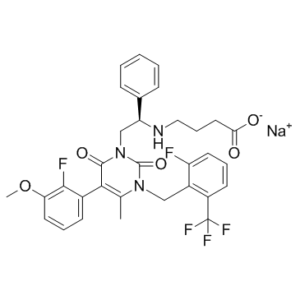
Elagolix sodium (NBI56418; ABT-620; Orilissa), the sodium salt of Elagolix, is an orally bioavailable and small molecule antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (KD = 54 pM) approved on 7/23/2018 by FDA for the management of moderate to severe pain associated with endometriosis. Elagolix is a short-acting GnRH antagonist that suppresses ovarian estrogen production in a dose-dependent manner, meaning that higher doses result in full suppression while lower doses only cause partial suppression. Elagolix's non-peptide structure and oral bioavailability make it the leader of a new class of GnRH inhibitors known as second-generation inhibitors.
Physicochemical Properties
| Molecular Formula | C32H29F5N3NAO5 | |
| Molecular Weight | 653.57 | |
| Exact Mass | 653.192 | |
| Elemental Analysis | C, 60.85; H, 4.79; F, 15.04; N, 6.65; O, 12.67 | |
| CAS # | 832720-36-2 | |
| Related CAS # | (R)-Elagolix; 834153-87-6; Elagolix-13C,d3 sodium | |
| PubChem CID | 24785956 | |
| Appearance | White to light yellow solid powder | |
| LogP | 4.591 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 11 | |
| Rotatable Bond Count | 12 | |
| Heavy Atom Count | 46 | |
| Complexity | 1090 | |
| Defined Atom Stereocenter Count | 1 | |
| SMILES | CC1N(CC2C(F)=CC=CC=2C(F)(F)F)C(=O)N(C[C@@H](C2C=CC=CC=2)NCCCC(=O)O)C(=O)C=1C1C=CC=C(OC)C=1F.[Na] |
|
| InChi Key | DQYGXRQUFSRDCH-UQIIZPHYSA-M | |
| InChi Code | InChI=1S/C32H30F5N3O5.Na/c1-19-28(21-11-6-14-26(45-2)29(21)34)30(43)40(18-25(20-9-4-3-5-10-20)38-16-8-15-27(41)42)31(44)39(19)17-22-23(32(35,36)37)12-7-13-24(22)33;/h3-7,9-14,25,38H,8,15-18H2,1-2H3,(H,41,42);/q;+1/p-1/t25-;/m0./s1 | |
| Chemical Name | sodium;4-[[(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-4-methyl-2,6-dioxopyrimidin-1-yl]-1-phenylethyl]amino]butanoate | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | GnRHR ( IC50 = 0.25 nM ); GnRHR ( Ki = 3.7 nM ) | |
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay | The competitive displacement of the radioligand GnRH from membranes prepared from HEK293 cells stably transfected with the full-length human GnRH receptor determines Elagolix sodium's affinity for hGnRH-R. | |
| Cell Assay | The human cloned GnRH receptor-expressing RBL-1 cells are seeded into 96-well plates at a density of 15,000 cells per well using inositol-free DMEM that contains 10% dialyzed FBS, 10 mM HEPES, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 50 μg/mL penicillin/streptomycin. The cells are labeled with 0.2 μCi myo-2-[3H]inositol for 48 hours. The assay buffer (140 mM NaCl, 4 mM KCl, 20 mM HEPES, 8.3 mM glucose, 1 mM MgCl2, 1 mM CaCl2, 10 mM LiCl, and 0.1% BSA) is applied to the cells for 15–60 minutes at 37 °C after they have been initially cleaned in PBS. Following GnRH (6 nM) stimulation, the cells are either left without an antagonist or exposed to it for 60 minutes at 37°C. After extracting the cells using 10 mM formic acid at 4°C for 30 minutes, the lysate is put onto Millipore plates that have 20 μg of Dowex AG1-X8 resin on them. Inositol phosphates are eluted with 1 M ammonium formate/0.1 M formic acid after the plate has been cleaned once in H2O and once in 60 mM ammonium formate/5 mM sodium tetraborate. A Lumaplate is used to transfer the eluate, and a TopCount NXT is used to count it. | |
| Animal Protocol |
Rhesus Monkey 10 and 50 mg/kg (oral); 3 and 10 mg/kg (i.v.) p.o. or i.v. |
|
| References |
[1]. J Med Chem . 2008 Dec 11;51(23):7478-85. |
|
| Additional Infomation | See also: Elagolix (has active moiety) ... View More ... |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (3.83 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (3.83 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (3.83 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 50 mg/mL (76.50 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5301 mL | 7.6503 mL | 15.3006 mL | |
| 5 mM | 0.3060 mL | 1.5301 mL | 3.0601 mL | |
| 10 mM | 0.1530 mL | 0.7650 mL | 1.5301 mL |