Physicochemical Properties
| Molecular Formula | C21H19NO4 |
| Molecular Weight | 349.38 |
| Exact Mass | 349.131 |
| Elemental Analysis | C, 72.19; H, 5.48; N, 4.01; O, 18.32 |
| CAS # | 6880-91-7 |
| PubChem CID | 485077 |
| Appearance | White to off-white solid |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 565.9±50.0 °C at 760 mmHg |
| Flash Point | 171.6±27.3 °C |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.657 |
| LogP | 4.56 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 26 |
| Complexity | 516 |
| Defined Atom Stereocenter Count | 0 |
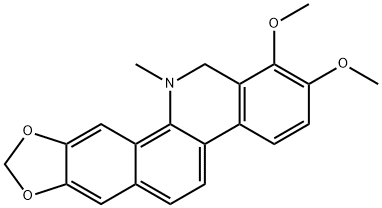
| SMILES | O1C([H])([H])OC2=C1C([H])=C1C(=C2[H])C([H])=C([H])C2C3C([H])=C([H])C(=C(C=3C([H])([H])N(C([H])([H])[H])C=21)OC([H])([H])[H])OC([H])([H])[H] |
| InChi Key | ALZAZMCIBRHMFF-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C21H19NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-9H,10-11H2,1-3H3 |
| Chemical Name | 1,2-dimethoxy-12-methyl-12,13-dihydro-[1,3]dioxolo[4',5':4,5]benzo[1,2-c]phenanthridine |
| Synonyms | 12,13-Dihydrochelerythrine; Dihydrochelerythrine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Natural product; antifungal |
| ln Vitro | A quaternary benzo[c]phenanthridine alkaloid chelerythrine displays a wide range of biological activities including cytotoxicity to normal and cancer cells. In contrast, less is known about the biological activity of dihydrochelerythrine, a product of chelerythrine reduction. We examined the cytotoxicity of chelerythrine and dihydrochelerythrine in human promyelocytic leukemia HL-60 cells. After 4h of treatment, chelerythrine induced a dose-dependent decrease in the cell viability with IC50 of 2.6 microM as shown by MTT reduction assay. Dihydrochelerythrine appeared to be less cytotoxic since the viability of cells exposed to 20 microM dihydrochelerythrine for 24h was reduced only to 53%. Decrease in the viability induced by both alkaloids was accompanied by apoptotic events including the dissipation of mitochondrial membrane potential, activation of caspase-9 and -3, and appearance of cells with sub-G1 DNA. Moreover, chelerythrine, but not dihydrochelerythrine, elevated the activity of caspase-8. A dose-dependent induction of apoptosis and necrosis by chelerythrine and dihydrochelerythrine was confirmed by annexin V/propidium iodide dual staining flow cytometry. Besides, both alkaloids were found to induce accumulation of HL-60 cells in G1 phase of the cell cycle. We conclude that both chelerythrine and dihydrochelerythrine affect cell cycle distribution, activate mitochondrial apoptotic pathway, and induce apoptosis and necrosis in HL-60 cells[2]. |
| ln Vivo | The antifungal activities of dihydrosanguinarine and dihydrochelerythrine, isolated from the leaves of Macleaya microcarpa, were evaluated on 12 plant pathogenic fungi; the two compounds exhibited the highest antifungal activity against Botrytis cinerea Pers. Among the 11 tested plant pathogenic fungi in vitro, the two compounds showed the highest antifungal activity against B. cinerea Pers, with 95.16% and 98.32% mycelial growth inhibition at 50 µg mL⁻¹, respectively. In addition, the two compounds inhibited spore germination in vitro in a concentration-dependent manner. They also showed potent protective and curative effects against Erysiphe graminis and B. cinerea in vivo. This is the first report on the antifungal activity of dihydrosanguinarine and dihydrochelerythrine against pathogenic plant fungi[1]. |
| References |
[1]. Inhibitory activity of dihydrosanguinarine and dihydrochelerythrine against phytopathogenic fungi. Nat Prod Res. 2011 Jul;25(11):1082-9. [2]. Chelerythrine and dihydrochelerythrine induce G1 phase arrest and bimodal cell death in human leukemia HL-60 cells. Toxicol In Vitro. 2008 Jun;22(4):1008-17. [3]. Antibacterial compounds from Zanthoxylum rhetsa. Arch Pharm Res. 2012 Jul;35(7):1139-42. |
| Additional Infomation |
Dihydrochelerythrine is a benzophenanthridine alkaloid. Dihydrochelerythrine has been reported in Zanthoxylum simulans, Zanthoxylum ailanthoides, and other organisms with data available. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~20.83 mg/mL (~59.62 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8622 mL | 14.3111 mL | 28.6221 mL | |
| 5 mM | 0.5724 mL | 2.8622 mL | 5.7244 mL | |
| 10 mM | 0.2862 mL | 1.4311 mL | 2.8622 mL |