Physicochemical Properties
| Molecular Formula | C26H25F3N8O |
| Molecular Weight | 522.524914503098 |
| Exact Mass | 522.21 |
| CAS # | 1639009-81-6 |
| Related CAS # | CD532 hydrochloride;2926498-81-7 |
| PubChem CID | 77232197 |
| Appearance | White to off-white solid powder |
| LogP | 5.7 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Heavy Atom Count | 38 |
| Complexity | 759 |
| Defined Atom Stereocenter Count | 0 |
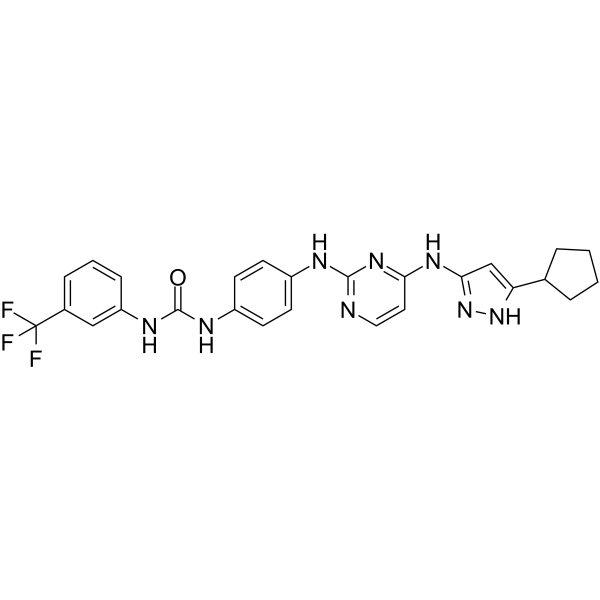
| SMILES | FC(C1C=CC=C(C=1)NC(NC1C=CC(=CC=1)NC1=NC=CC(=N1)NC1C=C(C2CCCC2)NN=1)=O)(F)F |
| InChi Key | GBMIFBVLJSCVJT-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C26H25F3N8O/c27-26(28,29)17-6-3-7-20(14-17)33-25(38)32-19-10-8-18(9-11-19)31-24-30-13-12-22(35-24)34-23-15-21(36-37-23)16-4-1-2-5-16/h3,6-16H,1-2,4-5H2,(H2,32,33,38)(H3,30,31,34,35,36,37) |
| Chemical Name | 1-[4-[[4-[(5-cyclopentyl-1H-pyrazol-3-yl)amino]pyrimidin-2-yl]amino]phenyl]-3-[3-(trifluoromethyl)phenyl]urea |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Aurora A 45 nM (IC50) |
| ln Vitro | In MYCN-amplified neuroblastoma cell lines SK-N-BE(2) and Kelly, CD532 (1-10000 nM; 72 h) is cytotoxic, with EC50s of 223.2 nM and 146.7 nM, respectively[1]. MYCN protein in SK-N-BE(2) cells is lost in a dose-dependent manner upon exposure to CD532 (0.1–1 μM; 24 h)[1]. SK-N-BE(2) cells are prevented from entering S-phase by CD532 (1 μM; 6 h)[1]. |
| ln Vivo | In mice with subcutaneous sonic hedgehog (SHH)-subtype medulloblastoma, CD532 (25 mg/kg; ip twice weekly for 3 weeks) reduces the tumor volume and improves survival[1]. MYCN-amplified neuroblastoma xenografts produce less MYCN protein when treated with CD532 (60 mg/kg; intraperitoneally for two days)[1]. In mice, CD532 (20 mg/kg; ip) exhibits an AUC0-24 of 27 μM·h and a serum half-life of approximately 1.5 hours[1]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: SK-N-BE(2) and Kelly cells Tested Concentrations: 1, 10, 100, 1000, 10000 nM Incubation Duration: 72 hrs (hours) Experimental Results: Inhibited the cell viability of SK-N-BE(2) and Kelly cells, with EC50s of 223.2 nM and 146.7 nM, respectively. Cell Cycle Analysis[1] Cell Types: SK-N-BE(2) cells Tested Concentrations: 1 μM Incubation Duration: 4 hrs (hours) Experimental Results: Resulted in a rapid and potent loss of S-phase and accumulation in both G0/G1 and G2. Western Blot Analysis[1] Cell Types: SK-N-BE(2) cells Tested Concentrations: 0.1, 0.25, 0.5, 1 μM Incubation Duration: 2, 4, 6, 24 hrs (hours) Experimental Results: Causes dose-dependent and time-dependent loss of MYCN protein. |
| Animal Protocol |
Animal/Disease Models: Homozygous nu/nu (nude) mice with SHH-subtype MYCN-expressing medulloblastoma[1] Doses: 25 mg/kg Route of Administration: Ip twice weekly for 3 weeks Experimental Results: diminished the level of MYCN protein and tumor volume and increases survival. |
| References |
[1]. Drugging MYCN through an allosteric transition in Aurora kinase A. Cancer Cell. 2014 Sep 8;26(3):414-427. [2]. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell. 2016 Apr 11;29(4):536-547. |
| Additional Infomation | CD532 is a member of the class of phenylureas that is urea with 4-[[4-[(5-cyclopentylpyrazol-3-yl)amino]pyrimidin-2-yl]amino]phenyl and 3-[3-(trifluoromethyl)phenyl substituents at positions N1 and N3. It has a role as an antineoplastic agent. It is an aminopyrimidine, a secondary amino compound, a member of pyrazoles, a member of cyclopentanes and a member of phenylureas. |
Solubility Data
| Solubility (In Vitro) | DMSO : 250 mg/mL (478.45 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.98 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.98 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (3.98 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9138 mL | 9.5690 mL | 19.1380 mL | |
| 5 mM | 0.3828 mL | 1.9138 mL | 3.8276 mL | |
| 10 mM | 0.1914 mL | 0.9569 mL | 1.9138 mL |