| Bioactivity | Omberacetam (GVS-111) is a medication promoted and prescribed in Russia and neighbouring countries as a nootropic. |
| Invitro | Nooglutil exhibits pharmacologically significant competition with a selective agonist of AMPA receptors ([G-3H]Ro 48-8587) for the receptor binding sites (with IC50 = 6.4 +/- 0.2 microM), while the competition of noopept for these receptor binding sites was lower by an order of magnitude (IC50 = 80 +/- 5.6 microM) [1]. GVS-111 significantly increased neuronal survival after H(2)O(2)-treatment displaying a dose-dependent neuroprotective activity from 10 nM to 100 microM, and an IC(50) value of 1.21+/-0.07 microM. GVS-111 inhibited the accumulation of intracellular free radicals and lipid peroxidation damage in neurons treated with H(2)O(2) or FeSO(4), suggesting an antioxidant mechanism of action . |
| In Vivo | N-Phenylacetyl-L-prolylglycine ethyl ester (GVS-111) administered intravenously at a dose of 0.5 mg/kg/day, for the first time 1 h after ischaemic lesion and then for 9 post-operative days, with the last administration 15 min before testing, attenuated the deficit [3]. GVS-111 itself was not found in rat brain 1 h after 5 mg/kg i.p. administration up to limit of detection (LOD) under high performance liquid chromatography (HPLC) conditions [4]. The most pronounced antiinflammatory effect of dipeptide was observed on the model of adjuvant arthritis in rats, where the drug administered over 25 days in a daily dose of 0.5 mg/kg (i.m.) or 5 mg/kg (p.o.) significantly reduced the chronic immune inflammation (on the 12th day, by 94.0 and 74.1%, respectively) . |
| Name | Noopept |
| Synonyms | 1-(2-Phenylacetyl)-L-prolyl-glycine ethyl ester;GVS 111;N-Phenylacetyl-L-prolylglycine ethyl ester;SGS 111;Ethyl N-[1-(Phenylacetyl)-L-prolyl]glycinat |
| CAS | 157115-85-0 |
| Formula | C17H22N2O4 |
| Molar Mass | 318.37 |
| InChI | InChI=1S/C17H22N2O4/c1-2-23-16(21)12-18-17(22)14-9-6-10-19(14)15(20)11-13-7-4-3-5-8-13/h3-5,7-8,14H,2,6,9-12H2,1H3,(H,18,22)/t14-/m0/s1 |
| Structure | 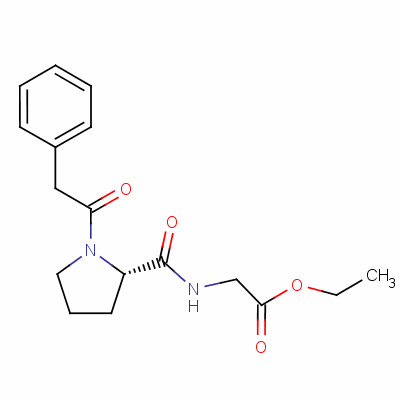 |
| Density | 1.202g/cm3 |
| Melting Point | 94.0 to 98.0 °C |
| Boiling Point | 547.3°C at 760 mmHg |
| Flash Point | 284.8±30.1 °C |
| Water Solubility | |
| Vapor Pressure | 0.0±1.5 mmHg at 25°C |
| Refractive Index | 1.549 |
| Specific Rotation | -116° (C=0.4,CHCl3) |
| Use | AMPA and NMDA receptor stimulator, NGF and BDNF protein stimulator |
| Safety Description | |
| Risk Codes | |
| Hazard Symbols | |
| Storage | Minimize open air exposure, store in a cool dry place |
| Reference | [1]. Firstova IuIu, et al. Studying specific effects of nootropic drugs on glutamate receptors in the rat brain. Eksp Klin Farmakol. 2011;74(1):6-10. [Content Brief] [2]. Pelsman A, et al. GVS-111 prevents oxidative damage and apoptosis in normal and Down's syndrome human cortical neurons. Int J Dev Neurosci. 2003 May;21(3):117-24. [Content Brief] [3]. Ostrovskaya RU, et al. Memory restoring and neuroprotective effects of the proline-containing dipeptide, GVS-111, in a photochemical stroke model. Behav Pharmacol. 1999 Sep;10(5):549-53. [Content Brief] [4]. Gudasheva TA, et al. The major metabolite of dipeptide piracetam analogue GVS-111 in rat brain and its similarity to endogenous neuropeptide cyclo-L-prolylglycine. Eur J Drug Metab Pharmacokinet. 1997 Jul-Sep;22(3):245-52. [Content Brief] [5]. Kovalenko LP, et al. Anti-inflammatory properties of noopept (dipeptide nootropic agent GVS-111). Eksp Klin Farmakol. 2002 Mar-Apr;65(2):53-5. [Content Brief] [6]. Kovalenko LP, et al. Preclinical study of noopept toxicity. Eksp Klin Farmakol. 2002 Jan-Feb;65(1):62-4. [Content Brief] |
