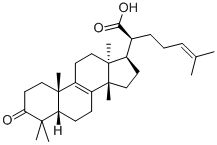
β-Elemonic acid is a novel and potent naturally occurring triterpene, isolated from Boswellia carterii. β-Elemonic acid is an inhibitor of prolyl endopeptidase. The anti-inflammatory and anticancer properties of elemonic acid are also evident. Additionally, β-Elemonic acid can result in the production of ROS, cell apoptosis, and COX-2 expression.
Physicochemical Properties
| Molecular Formula | C₃₀H₄₆O₃ |
| Molecular Weight | 454.68 |
| Exact Mass | 454.344 |
| CAS # | 28282-25-9 |
| Related CAS # | 28282-25-9 |
| PubChem CID | 24721570 |
| Appearance | White to off-white solid |
| Density | 1.07 |
| Boiling Point | 565.2±50.0 °C at 760 mmHg |
| Melting Point | 216-219 ºC |
| Flash Point | 309.6±26.6 °C |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.542 |
| Source | Triterpene from Boswellia carterii |
| LogP | 8.56 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 33 |
| Complexity | 904 |
| Defined Atom Stereocenter Count | 6 |
| SMILES | O=C1C([H])([H])C([H])([H])[C@@]2(C([H])([H])[H])C([H])(C1(C([H])([H])[H])C([H])([H])[H])C([H])([H])C([H])([H])C1=C2C([H])([H])C([H])([H])[C@@]2(C([H])([H])[H])C([H])(C([H])(C(=O)O[H])C([H])([H])C([H])([H])/C(/[H])=C(\C([H])([H])[H])/C([H])([H])[H])C([H])([H])C([H])([H])[C@@]21C([H])([H])[H] |
| InChi Key | XLPAINGDLCDYQV-SDTWUMECSA-N |
| InChi Code | InChI=1S/C30H46O3/c1-19(2)9-8-10-20(26(32)33)21-13-17-30(7)23-11-12-24-27(3,4)25(31)15-16-28(24,5)22(23)14-18-29(21,30)6/h9,20-21,24H,8,10-18H2,1-7H3,(H,32,33)/t20-,21-,24-,28+,29-,30+/m0/s1 |
| Chemical Name | (2S)-6-methyl-2-[(5R,10S,13S,14S,17S)-4,4,10,13,14-pentamethyl-3-oxo-1,2,5,6,7,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]hept-5-enoic acid |
| Synonyms | βElemonic acid; β Elemonic acid |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | COX-2 |
| ln Vitro | In human A549 lung cancer cells, β-elemonic acid (1, 3, 10, 20 µM; 24 hours) significantly promotes apoptosis in a time- and dose-dependent manner [1]. Human NSCLC A549 cells are subjected to strong cytotoxic effects from β-elemonic acid (1, 3, 10, 20 µM; 24 hours) that are dose-dependent. Following a 24-hour exposure to beta-citrate, the IC50 value is 6.92 µM[1]. G0/G1 phase cell percentage at 24 hours with 20 µM beta-citrate was 58.01%[1]. In A549 cells, β-elemonic acid (1, 3, 10, 20 µM; 24 hours) suppresses p42/44, MAPK/JNK, and p38 phosphorylation [1]. |
| ln Vivo | EA significantly suppressed the growth of transplanted colorectal tumors in nude mice [3]. |
| Enzyme Assay |
Xanthine Oxidase Inhibitory Assay [1]. Xanthine oxidase (XO) (EC 1.1.3.22) inhibition activity was assayed in phosphate buffer (0.1 M, pH 7.5, 250 μL) and XO (0.003 unit/well, 20 μL), and the test sample in 10 μL of DMSO was diluted to the desired range of concentrations, mixed in a 96-well microplate, and preincubated for 10 min at room temperature. The reaction was initiated by adding 20 μL of 0.1 mM xanthine. The uric acid formation was measured spectrophotometrically at 295 nm by using a microtiter plate reader (Molecular Devices, Spectramax 384). Phosphodiesterase I Inhibitory Assay[1]. Activity against phosphodiesterase I (Sigma P 4631) (EC 3.1.4.1) was assayed by using the reported method with the following modifications: 33 mM tris-HCl buffer pH 8.8, 30 mM Mg(C2H3O2)2·4H2O with 0.000742 U/well final concentration using microtiter plate assay, and 0.33 mM bis(p-nitrophenyl) phosphate (Sigma N-3002) as a substrate. Cystein and EDTA were used as positive controls (IC50 = 748 ± 15.00 and 274 ± 7.00 μM, respectively). After 30 min of incubation, the enzyme activity was monitored spectrophotometrically at 37 °C on a microtiter plate reader by following the release of p-nitrophenyl phosphate at 410 nm. Assays were conducted in triplicate. PEP Inhibitory Activity[1]. Prolyl endopeptidase (EC 3.4.21.26) was purchased from Seikagaku Corporation (Tokyo, Japan). N-Benzyloxycarbonyl-Gly-Pro-pNA and bacitracin were purchased from BACHEM Fine Chemicals Co. and Sigma Co., Ltd., respectively. PEP inhibitory activities were measured by a method developed by Yoshimoto 21 et al. and described in our previous publications. |
| Cell Assay |
Colony Formation CRC cells were cultured in 12-well plates at 37°C with 5% CO2 for 12 h until the cells adhered to the wall. Next, they were treated with 0–20 μg/ml of EA for 10 days. The cells were stained using crystal violet solution according to the manufacturer’s instructions. Cell Migration Assay Cell migration assay was performed to check the inhibitory effect of EA on migration of CRC cells. Cells were seeded at 40% confluence into 12-well plates around culture inserts and incubated at 37°C with 5% CO2. After 12 h, the inserts were removed, and the suspension cells were washed with phosphate-buffered saline (PBS). Fresh medium supplemented with 0–20 μg/ml EA was added. After 24 h incubation period, the width of cells scratches was observed under a microscope, and images were captured. DNA Synthesis Assay DNA synthesis was examined using the EDU-594 Cell Proliferation Assay Kit, according to the manufacturer’s instructions, and staining results were recorded under a fluorescence microscope |
| Animal Protocol |
Xenografts The mice experiment was approved by the ethics committee of Jining medical university, China. Female BALB/c nude mice were subcutaneously injected with 5 × 106 SW480 cells and randomly divided into two groups (n = 5 in each group). When the tumor reached a volume of 200 mm3, the mice were intraperitoneally injected with 10 mg/kg of EA (treatment group) or DMSO (vehicle) every 2 days for 24 days. All mice were euthanatized with CO2 at 24 days post-injection, and their tumors, kidneys, livers, and hearts were placed in 10% formalin for fixation. Hematoxylin and Eosin, Immunochemistry, and Immunofluorescence Staining Mouse tissues were fixed with 10% formalin, dehydrated, and embedded into paraffin blocks. The paraffin-embedded specimens were sectioned at a thickness of 4 μm using a microtome. The sections were dewaxed and stained with H&E. After being dewaxed and antigen repaired, the sections were stained for IHC and IF staining. The primary antibodies used in IHC were Rabbit-anti Ki67 and Rabbit-anti FTL (1:100; Proteintech, China). The secondary antibodies and 3, 3′-diaminobenzidine were purchased from Boster Bio. In IF staining, Rabbit-LC3 (1:250, Proteintech) was used as the primary antibody, and Goat-anti rabbit IgG H&L (Alexa Fluor 488, 1:400; Abcam) was used as the secondary antibody. |
| References |
[1]. Bioactive constituents from Boswellia papyrifera. J Nat Prod. 2005 Feb;68(2):189-93. [2]. β-Elemonic acid inhibits the cell proliferation of human lung adenocarcinoma A549 cells: The roleof MAPK, ROS activation and glutathione depletion. Oncol Rep. 2016 Jan;35(1):227-34. |
| Additional Infomation |
Beta-Elemonic acid is a triterpenoid. (2S)-6-methyl-2-[(5S,10S,13S,14S,17R)-4,4,10,13,14-pentamethyl-3-oxo-1,2,5,6,7,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]hept-5-enoic acid has been reported in Boswellia papyrifera with data available. |
Solubility Data
| Solubility (In Vitro) |
DMSO: 25~35 mg/mL (55~77 mM) H2O: < 0.1 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.50 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1993 mL | 10.9967 mL | 21.9935 mL | |
| 5 mM | 0.4399 mL | 2.1993 mL | 4.3987 mL | |
| 10 mM | 0.2199 mL | 1.0997 mL | 2.1993 mL |