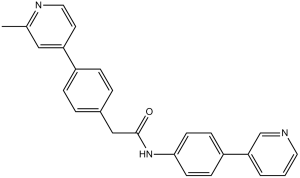
Wnt-C59 (also called Wnt-C 59; C59; Wnt-C-59; C-59) is a novel, highly potent and orally bioactive PORCN (porcupine) inhibitor with potential antineoplastic activity. It inhibits Wnt3A-mediated activation of a multimerized TCF-binding site driving luciferase with an IC50 of 74 pM in HEK293 cells. Wnt-C59 has a different selectivity with the reported Wnt inhibitor crizotinib. When tested with HT1080 and Hela cells, Wnt-C59 showed inhibition on activation of a multimerized TCF-binding site driving luciferase mediated by Wnt3A by completely abrogating Wnt secretion. Wnt-C59 displays good bioavailability in mice. Wnt-C59 blocks progression of mammary tumors in MMTV-WNT1 transgenic mice while downregulating Wnt/β-catenin target genes. Wnt-C59 has the potential to eradicate cancer stem cells in human tumors. Wnt-C59 inhibits stemness properties of NPC cells in a dosage-dependent manner by arresting sphere formation in both HNE1 and SUNE1 cells.
Physicochemical Properties
| Molecular Formula | C25H21N3O | |
| Molecular Weight | 379.45 | |
| Exact Mass | 379.168 | |
| Elemental Analysis | C, 79.13; H, 5.58; N, 11.07; O, 4.22 | |
| CAS # | 1243243-89-1 | |
| Related CAS # | Wnt-C59;1243243-89-1 | |
| PubChem CID | 57519544 | |
| Appearance | White to yellow solid | |
| Density | 1.2±0.1 g/cm3 | |
| Boiling Point | 628.3±55.0 °C at 760 mmHg | |
| Flash Point | 333.8±31.5 °C | |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C | |
| Index of Refraction | 1.648 | |
| LogP | 4.19 | |
| Hydrogen Bond Donor Count | 1 | |
| Hydrogen Bond Acceptor Count | 3 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 29 | |
| Complexity | 508 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | O=C(NC1=CC=C(C2=CC=CN=C2)C=C1)CC3=CC=C(C4=CC(C)=NC=C4)C=C3 |
|
| InChi Key | KHZOJCQBHJUJFY-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C25H21N3O/c1-18-15-22(12-14-27-18)20-6-4-19(5-7-20)16-25(29)28-24-10-8-21(9-11-24)23-3-2-13-26-17-23/h2-15,17H,16H2,1H3,(H,28,29) | |
| Chemical Name | 2-(4-(2-methylpyridin-4-yl)phenyl)-N-(4-(pyridin-3-yl)phenyl)acetamide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | PORCN (IC50 = 74 pM)[1] | ||
| ln Vitro |
As determined by blocking Wnt palmitoylation, Wnt interaction with the carrier protein Wntless/WLS, Wnt secretion, and Wnt activation of β-catenin reporter activity, Wnt-C59 (C59) reduces PORCN function in vitro at nanomolar concentrations. With an IC50 of 74 pM, Wnt-C59 blocks WNT3A-mediated activation of a multimerized TCF-binding site that drives luciferase[1]. Wnt-C59 (C59) is a potent inhibitor of PORCN enzymatic activity [1] The small-molecule 2-(4-(2-methylpyridin-4-yl)phenyl)-N-(4-(pyridin-3-yl)phenyl) acetamide was recently developed and patented by Novartis as a Wnt signaling modulato. It is commercially available under the name C59 from at least 2 sources, and is claimed to inhibit PORCN enzyme activity at nanomolar concentrations. However, there is no peer-reviewed published information on its efficacy and molecular target. Because a potent, bioavailable, and stable PORCN inhibitor is not yet available we evaluated C59. We find that C59 indeed functions as a bona fide PORCN inhibitor using a number of cell-based assays. C59 inhibits WNT3A-mediated activation of a multimerized TCF-binding site driving luciferase (Super8xTopFlash; STF) with an IC50 of 74 pmol/L (Fig. 1A). As expected for a PORCN inhibitor, Wnt secretion into culture medium is completely abrogated by C59 treatment (Fig. 1A, inset). Consistent with C59 targeting PORCN, overexpression of PORCN rescues the inhibition of WNT3A-mediated STF activity, similar to that of an unrelated PORCN inhibitor IWP-1 (refs. 21, 22; Fig. 1B). Wnt acylation is required for binding to the carrier protein WLS. WNT3A and WNT8A coimmunoprecipitate with WLS, but this interaction is blocked when cells have been pretreated with C59 (Fig. 1C). Using alkyne palmitic acid and click chemistry, we find that C59 prevents incorporation of palmitate into WNT3A, consistent with inhibition of PORCN activity (Fig. 1D). C59 inhibits the activity of all splice variants of murine PORCN (Fig. 2A). In preliminary studies, we found that very high concentrations of C59 were required to produce developmental phenotypes in Xenopus embryogenesis. Consistent with this, while Xenopus laevis PORCN was active when expressed in PORCN-null human cells, its activity was resistant to inhibition by C59 (Fig. 2A). Because the Xenopus protein is 77% identical to human PORCN, this provides genetic evidence that PORCN is the molecular target of C59, suggests a mechanism for C59 drug resistance to emerge, and indicates that less related MBOAT proteins would also be unaffected by C59. Showing that inhibition of PORCN is likely to prevent all Wnt-mediated signaling, we found that 9 of 9 β-catenin activating Wnts and 4 of 4 additional noncanonical Wnts lost activity when cells were treated with C59 (Fig. 2B and C). In summary, C59 is a nanomolar inhibitor of mammalian PORCN acyltransferase activity and blocks activation of all evaluated human Wnts. Thus, we anticipate that C59 administration will prevent all human and murine Wnt-dependent signaling. SUNE1 cells exhibited a strong tolerance to Wnt-C59 (C59), as reported in many other human cancer cell lines. Both CNE1 and HNE1 cells showed reduced growth abilities in the higher Wnt-C59 concentration (20 μM), while HK1 cells were sensitive to all concentrations (5 μM, 10 μM, and 20 μM) of Wnt-C59 treatments. Both SUNE1 and HNE1 cells showed a greater ability to form spheres (Figures 1A and 1B). Their IC50 for Wnt-C59 treatment at 48 hours is greater than 60 μM. Additionally, these two cell lines have obviously different in vivo cell growth dynamics, providing good animal models to determine biological effects of Wnt-C59 in NPC cells. A low concentration (1 μM) of Wnt-C59 (C59) treatment suppresses SUNE1 cells and 3D growth was clearly inhibited compared to control untreated cells (Figure 3A). The inhibitory effects of Wnt-C59 were dosage-dependent in these NPC cells; both 5 μM and 20 μM Wnt-C59 treatments can arrest sphere formation in both HNE1 and SUNE1 cell lines, but not CNE1 cells. However, cells showing a monolayer growth with Wnt-C59 treatments were continuously expanding and both 3D inhibition and monolayer growth were consistent, as observed from weeks 1 to 3 (Figure 3B). After a three-week treatment, we withdrew Wnt-C59 (C59) and continued to culture cells for 10 to 20 days more under control cell culture conditions. No obvious 3D growth resumed or was detected in HNE1 cells, although cell densities and incubation periods were sufficient to generate 3D growth for untreated cells. However, monolayer-growing SUNE1 cells gradually formed huge spheres in 20 days under untreated culture conditions (Figure 3C). These findings indicated that inhibitory effects of Wnt-C59 on stemness were irreversible as seen in HNE1. The HNE1 cells with stemness properties in the whole cell populations could be safely eradicated by the Wnt inhibitor. |
||
| ln Vivo |
In mice, wnt-C59 exhibits good bioavailability. Wnt-C59 downregulates Wnt/β-catenin target genes while preventing the growth of breast cancers in MMTV-WNT1 transgenic mice[1]. In human cancers, Wnt-C59 has the ability to eliminate cancer stem cells. Wnt-C59 stops the creation of spheres in both HNE1 and SUNE1 cells, which suppresses the stemness characteristics of NPC cells in a dosage-dependent manner[2].
Wnt-C59 (C59) can be administered to mice and prevents tumor growth [1] To test the role of Wnt signaling in vivo, we assessed the bioavailability and in vivo half-life of C59 in mice. After either intravenous (2.5 mg/kg) or oral administration (5 mg/kg), the compound half-life in blood was approximately 1.94 hours. Notably, C59 concentration remained greater than 10-fold above the in vitro IC50 for at least 16 hours following a single oral dose (Fig. 3A). On the basis of the pharmacokinetic profiling, C59 was administered once daily to test its efficacy in treating established Wnt-driven tumors. In mice carrying a mouse mammary tumor virus (MMTV)-WNT1 transgene, overexpression of murine WNT1 causes a high incidence of mammary adenocarcinomas beginning at 10 weeks of age. Notably, tumors arising in these mice remain Wnt dependent but have diverse molecular phenotypes and growth rates consistent with the hypothesis that WNT1 expands a vulnerable population that then undergoes second hits. To test the in vivo efficacy of Wnt-C59 (C59), we transplanted fragments from 2 independent primary MMTV-WNT1 tumors orthotopically into nude mice. Following development of palpable tumors, mice were treated with either vehicle or C59, 10 mg/kg/d for 17 days. C59 administration arrested or reversed tumor growth in all treated mice (n = 22; Fig. 3B). After 17 days of treatment, the tumors were removed and further analyzed. Final tumor weights were significantly different (Fig. 3C). To confirm that C59 was active in immunocompetent mice, we monitored a colony of female nulliparous Bl6 MMTV-WNT1 mice for tumor development. When tumors became palpable, the mice were treated with either vehicle or C59 (5 mg/kg/d). While the number of mice enrolled in this study was smaller, again even the lower dose of C59 significantly blocked tumor growth (Fig. 3D). Final tumor weights are shown in Supplementary Fig. S2A. Tumor growth inhibition is associated with decreased Wnt/β-catenin signaling in tumors [1] To determine whether the inhibition of tumor growth was accompanied by inhibition of Wnt/β-catenin signaling, we examined the expression of selected target genes in the allograft and primary tumors by quantitative reverse-transcription PCR (qRT-PCR). Axin2, Ccnd1, c-Myc, and Tcf7 transcripts were significantly reduced in tumors from mice treated with C59 (Fig. 4A and Supplementary Fig. S2B). Consistent with a decrease in c-Myc and CyclinD, treated tumors also had significantly decreased proliferation as indicated by Ki67 staining (Fig. 4B). Wnt-C59 (C59) inhibits the Wnt pathway in vivo [2] Wnt signaling in the stroma plays a vital role in the stem cell niche, as reported recently. Therefore, we examined protein expression of active β-catenin and Axin2 in tumor tissues. These two proteins clearly accumulated in stromal cells around cancer nests, especially in the fast-growing region of the tumor tissues. No obvious difference of expression of these two proteins was detected between control and Wnt-C59-treated mice with injected SUNE1 cells, indicating that the tumor microenvironment in SUNE1 (Figure 4A) and HNE1 cells (Figure 4B) had favorable and up-regulated Wnt pathway activities. The Wnt-C59 had limited influence on these growing tumors in treated mice with injected SUNE1 cells. Importantly, expression of active β-catenin and Axin2 in liver (Figure 4C) and kidney (Figure 4D) was clearly down-regulated in the Wnt-C59-treated HNE1 cell-injected mice compared to control mice. These findings confirmed that the administration of Wnt-C59 generated a systemic influence on the Wnt pathway in treated animals within a 41-day assay period, which induced tumor suppression in tested mice. |
||
| Enzyme Assay |
Wnt Secretion, TOPFlash Assays, Dvl2 mobility shift, and PORCN Rescue[1] For determination of C59 IC50, STF3a cells were treated with compounds diluted 1/1000 into culture media, and luciferase activity measured 48 hrs later. To monitor Wnt secretion into culture media, STF3a cells were treated with C59 diluted in fresh 1% FBS containing media added at half volume. Lysates were collected in 4% SDS 24 hours later. For SuperTOPflash assays in HT1080 cells, experiments were transfected in 24-well plates with 50 ng Wnt, 100 ng mCherry, and 650 ng SuperTOPflash. For PORCN rescue experiments, 100 ng 3xHA-mPORCN-D was added. For measurement of Dvl2 mobility shift, transfection was with 200 ng of each Wnt plasmid and 600 ng mCherry. Lysates were prepared in 100 mM sodium phosphate, pH 7.5, 150 mM NaCl, 1% IGEPAL-CA630, complete protease inhibitor cocktail and Western blotting was performed to detect Dvl2. Wnt-WLS interaction [1] HeLa cells in 6 well dishes were transfected with C-terminal V5-tagged WNT3A or WNT8A. Six hours after transfection, the media was replaced with addition of either 0.1% DMSO as a control or C59 at a final concentration of 10 nM. Following overnight incubation, the cells were lysed with the buffer: 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40 and complete protease inhibitors). A total of 500 μg lysate was immunoprecipitated with the rabbit polyclonal antibody against the C-terminus of WLS. Western blotting was performed for V5 and WLS (using mouse antibody to WLS). Metabolic Labeling with Alk-C16 and Click Chemistry[1] HT1080 cells plated at a density of 3 x 106 cells in 10 cm plates were transfected with C-terminally V5-tagged WNT3A. After six hours of transfection the media was replaced with DMEM containing 5% fatty acid free BSA (Sigma) with or without 100 μM ω-alkynyl palmitic acid (Alk-C16), synthesized as previously reported (3,4). Cells were simultaneously treated with 0.1% DMSO as a control or 100 nM C59. Following overnight incubation, cells were washed twice with cold PBS and cells’ lysates were prepared in the buffer: 50mM HEPES, 150nM NaCl, 1mM EDTA, 1% NP-40 and protease inhibitors (Roche). The protein lysates were incubated overnight with anti-V5 antibody followed by addition of 30 μL of Protein A/G plus agarose (50% slurry) for 2 hours. Pellets were washed four times with lysis buffer and resuspended in sodium phosphate buffer (100 mM sodium phosphate, pH 7.5, 1% Nonidet P-40, 150mM NaCl). The immunoprecipitated proteins were then subjected to the click labeling reaction in a 50 μl volume for 1 hr RT at final concentrations of the following reagents added serially: 0.1 mM biotin-azide (Life Technologies), 1 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 0.2 mM Tris[(1-benzyl- 1H-1,2,3-triazol-4- yl)methyl]amine (TBTA) dissolved in DMSO/tert-butanol (20%/80%), and 1 mM CuSO4 (freshly prepared in water). Labeled immunoprecipitated proteins were resolved by SDS–PAGE, transferred to PVDF membrane, blotted with anti-V5 primary antibody followed by Dylight 680 conjugated anti-mouse antibody and Dylight 800 conjugated streptavidin, then visualized in respective channels |
||
| Cell Assay |
In vitro C59 Toxicity Studies[1] HCT116, K562, HL60, A549, U-2-OS, U266, HCC1937, HCC38, MCF7, BT-20, MB157, MDA-MB-435S, MDA-MB-231, MDA-MB-468, DLD1, SW480, T98G, A172, U-87MG, KATO III, SNU1, SNU5, SNU16, SK-MEL-5, SK-MEL-28, HS294T, A375, A2058, MEWO, FU97, IM95, IM95M, NUGC-3, NUGC-4, OCUM-1, SCH, MKN1, MKN7, MKN45, MKN74, AZ521, JHH2, SNU216, YCC-3 (Korean Cell Line Bank, Korea), EOL-1 were grown in the culture conditions as specified by suppliers. For the assay, 5000 cells in 75 μl culture medium were seeded in each well of black 96 well plates and incubated overnight at 37°C. C59 was serially diluted in medium as 4x stock and then 25 μl of each dilution was added to the cells to give the final concentration of 50 μM to 1.5 nM. After treatment for 2 days, 100 μl of CellTiter-Glo® Luminescent Cell Viability Assay reagent was added to each well and incubated for 10 minutes at room temperature. The luminescence was measured using Tecan Safire instrument. |
||
| Animal Protocol |
|
||
| References |
[1]. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013 Jan 15;73(2):502-7. [2]. Wnt-C59 arrests stemness and suppresses growth of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway in the tumor microenvironment. Oncotarget. 2015 Jun 10;6(16):14428-39. |
||
| Additional Infomation |
Porcupine (PORCN) is a membrane bound O-acyltransferase that is required for Wnt palmitoylation, secretion, and biologic activity. All evaluable human Wnts require PORCN for their activity, suggesting that inhibition of PORCN could be an effective treatment for cancers dependent on excess Wnt activity. In this study, we evaluated the PORCN inhibitor Wnt-C59 (C59), to determine its activity and toxicity in cultured cells and mice. C59 inhibits PORCN activity in vitro at nanomolar concentrations, as assessed by inhibition of Wnt palmitoylation, Wnt interaction with the carrier protein Wntless/WLS, Wnt secretion, and Wnt activation of β-catenin reporter activity. In mice, C59 displayed good bioavailability, as once daily oral administration was sufficient to maintain blood concentrations well above the IC(50). C59 blocked progression of mammary tumors in MMTV-WNT1 transgenic mice while downregulating Wnt/β-catenin target genes. Surprisingly, mice exhibit no apparent toxicity, such that at a therapeutically effective dose there were no pathologic changes in the gut or other tissues. These results offer preclinical proof-of-concept that inhibiting mammalian Wnts can be achieved by targeting PORCN with small-molecule inhibitors such as C59, and that this is a safe and feasible strategy in vivo. [1] In this study, we confirm that the small-molecule C59 is a nanomolar inhibitor of the acyltransferase activity of PORCN, and show that small-molecule–mediated inhibition of PORCN is an effective means for preventing WNT1-driven tumor growth in mice. C59 inhibits palmitoylation of Wnts and is not active against Xenopus PORCN. Thus, changes in the primary sequence of PORCN confer resistance to C59, confirming genetically that PORCN is the target of C59. C59 is more than100-fold more potent than the previously reported PORCN inhibitor IWP1. We find no apparent toxicity to cells or mice at a drug concentration that effectively inhibits MMTV-WNT1–driven tumor growth. Intestinal architecture of treated mice appears normal. A similar lack of intestinal toxicity was seen when Wnt signaling was inhibited with Fzd8CRD-Fc. We speculate that Wnt-addicted tumors are hypersensitive to small reductions in Wnt activity, whereas normal tissues such as intestine are more tolerant of decreases in Wnt signals and/or have alternative pathways for self-renewal. The Wnt pathways contribute to the progression of various cancers, via both β-catenin–activating mutations and by paracrine and autocrine Wnt signaling. Increased Wnt production has also been identified in diverse nonmalignant diseases. In many cases, the implicated Wnts may be working via non-β-catenin pathways. PORCN inhibitors may therefore have efficacy even in diseases without activated β-catenin. Thus, it is a longstanding goal to identify therapeutics that can effectively target this pathway. Our recent work has confirmed that PORCN is a key node for fine control of total Wnt-dependent cell signaling, further supporting its use as a target. As such, specific and bioavailable inhibitors of PORCN represent attractive new molecules that may be of value in the treatment of various cancers, in addition to other Wnt-stimulated diseases.[1] Wnt/β-catenin signaling is responsible for the generation of cancer stem cells (CSCs) in many human tumors, including nasopharyngeal carcinoma (NPC). Recent studies demonstrate that Wnt or PORCN inhibitor, Wnt-C59 (C59), inhibits tumor growth in MMTV-WNT1 transgenic mice. The effect of Wnt-C59 (C59) in human tumors is not clear. In this study, the NPC cell lines investigated manifest heterogeneous responses to Wnt-C59 treatment. Wnt-C59 decreased tumor growth of SUNE1 cells in mice immediately following the administration of Wnt-C59. Mice injected with HNE1 cells did not develop visible tumors after the treatment of Wnt-C59, while control mice developed 100% tumors. Wnt-C59 inhibited stemness properties of NPC cells in a dosage-dependent manner by arresting sphere formation in both HNE1 and SUNE1 cells. Thus, Wnt-C59 has the potential to eradicate CSCs in human tumors. Active β-catenin and Axin2 proteins were strongly expressed in stromal cells surrounding growing tumors, confirming the importance of Wnt signaling activities in the microenvironment being driving forces for cell growth. These novel findings confirm the ability of Wnt-C59 to suppress Wnt-driven undifferentiated cell growth in NPC. Both anti-Wnt signaling and anti-CSC approaches are feasible strategies in cancer therapy. [2] To detect long-term effects of Wnt-C59 in animals, which have not been reported yet, we investigated tumor growth of HNE1 cells. These cells require a longer latency period to form growing tumors in mice, reflecting greater intra-tumoral heterogeneity in this cell line. In the control group, surviving tumor cells, presumably CSCs, expanded in the injection sites and formed progressively growing tumors very quickly after the latency period. In contrast, injected tumor cells died and did not form visible and progressively growing tumors after the treatment of Wnt-C59 in animals. It was not possible to directly show the presence of CSCs in injected HNE1 cells, but the sphere inhibition assay demonstrates that Wnt-C59 could safely eliminate cells with stemness properties in an irreversible manner. Both in vitro and in vivo assays further confirm these findings and support the notion for the presence of CSCs in this cell line. Targeting CSCs to suppress tumor growth is a major focus in current cancer research. The current findings demonstrate that Wnt-C59 can effectively suppress Wnt signaling and stemness properties of certain tumor cells. We provide functional evidence demonstrating the use of an anti-CSC agent for tumor growth control is a feasible approach in cancer therapy and may be broadly exploited in human tumors.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.59 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.59 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (6.59 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 2% DMSO+30% PEG 300+5% Tween 80+ddH2O:5 mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6354 mL | 13.1770 mL | 26.3539 mL | |
| 5 mM | 0.5271 mL | 2.6354 mL | 5.2708 mL | |
| 10 mM | 0.2635 mL | 1.3177 mL | 2.6354 mL |