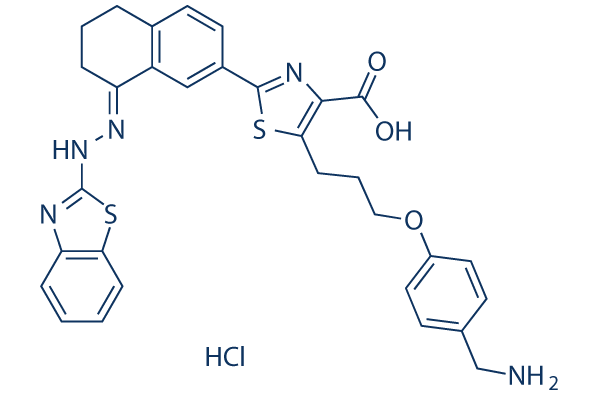
WEHI-539 hydrochloride, the HCl salt of WEHI-539, is a potent and selective inhibitor of BCL-XL with IC50 value of 1.1 nM. WEHI-539 increases the effects of carboplatin on caspase 3/7 activity, PARP cleavage, and annexin V labeling. In Ovcar-4 (5 μM in Ovcar-4) and Ovsaho (1 μM in Ovsaho) cells, WEHI-539 alone results in observable PARP cleavage. With IC50 and KD values near or below 1 nM and a slow dissociation rate, WEHI-539 not only binds to BCL-XL very tightly but also triggers apoptotic reactions that require BAX, BAK, or both. Additionally, its biological activity is linked to its binding profile because high levels of MCL-1, BCL-2, and BCL-W confer noticeable resistance. Additionally, its capacity to destroy platelets is a recognized indicator of on-target BCL-XL inhibition.
Physicochemical Properties
| Molecular Formula | C31H30CLN5O3S2 | |
| Molecular Weight | 620.184603214264 | |
| Exact Mass | 619.15 | |
| Elemental Analysis | C, 60.04; H, 4.88; Cl, 5.72; N, 11.29; O, 7.74; S, 10.34 | |
| CAS # | 2070018-33-4 | |
| Related CAS # | WEHI-539;1431866-33-9 | |
| PubChem CID | 78357795 | |
| Appearance | Light yellow to yellow solid powder | |
| Hydrogen Bond Donor Count | 4 | |
| Hydrogen Bond Acceptor Count | 10 | |
| Rotatable Bond Count | 10 | |
| Heavy Atom Count | 42 | |
| Complexity | 903 | |
| Defined Atom Stereocenter Count | 0 | |
| SMILES | C1CC2=C(C=C(C=C2)C3=NC(=C(S3)CCCOC4=CC=C(C=C4)CN)C(=O)O)/C(=N/NC5=NC6=CC=CC=C6S5)/C1.Cl |
|
| InChi Key | GJBYTVIQTMIXGA-DYICZVFTSA-N | |
| InChi Code | InChI=1S/C31H29N5O3S2.ClH/c32-18-19-10-14-22(15-11-19)39-16-4-9-27-28(30(37)38)34-29(40-27)21-13-12-20-5-3-7-24(23(20)17-21)35-36-31-33-25-6-1-2-8-26(25)41-31;/h1-2,6,8,10-15,17H,3-5,7,9,16,18,32H2,(H,33,36)(H,37,38);1H/b35-24+; | |
| Chemical Name | 5-[3-[4-(aminomethyl)phenoxy]propyl]-2-[(8E)-8-(1,3-benzothiazol-2-ylhydrazinylidene)-6,7-dihydro-5H-naphthalen-2-yl]-1,3-thiazole-4-carboxylic acid;hydrochloride | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bcl-xL (IC50 = 1.1 nM) | ||
| ln Vitro | NSC 241240-induced caspase 3/7 activity, PARP cleavage, and annexin V labeling are enhanced by WEHI-539. In Ovcar-4 (5 μM in Ovcar-4) and Ovsaho (1 μM in Ovsaho) cells, WEHI-539 alone significantly increases PARP cleavage[2]. | ||
| ln Vivo |
|
||
| Enzyme Assay |
The assay buffer (stock 50 mM HEPES and 100 mM NaCl, pH 7.5) is prepared fresh daily and adjusted to 5 mM DTT, casein (0.1 mg/mL sodium salt; aliquots stored at -20 °C) and Tween 20. The prosurvival BCL-2 family protein BCL-X(L) is often overexpressed in solid tumors and renders malignant tumor cells resistant to anticancer therapeutics. Enhancing apoptotic responses by inhibiting BCL-X(L) will most likely have widespread utility in cancer treatment and, instead of inhibiting multiple prosurvival BCL-2 family members, a BCL-X(L)-selective inhibitor would be expected to minimize the toxicity to normal tissues. We describe the use of a high-throughput screen to discover a new series of small molecules targeting BCL-X(L) and their structure-guided development by medicinal chemistry. The optimized compound, WEHI-539 (7), has high affinity (subnanomolar) and selectivity for BCL-X(L) and potently kills cells by selectively antagonizing its prosurvival activity. WEHI-539 will be an invaluable tool for distinguishing the roles of BCL-X(L) from those of its prosurvival relatives, both in normal cells and notably in malignant tumor cells, many of which may prove to rely upon BCL-X(L) for their sustained growth.[1] |
||
| Cell Assay | Ovcar-8, Ovcar-3, Ovcar-4, and Ovsaho cells are grown in the RPMI medium, Igrov-1, Cov-362 and Cov-318 cells are grown in DMEM, and Fuov-1 cells are grown in a DMEM/F-12 nutrient solution. DMSO is used to prepare a 20 mM solution of ABT-737, ABT-199, and WEHI-539. Cells are plated in 96-well plates for cell growth assays (5,000 cells/well for all cell lines, 2,500 cells/well for Ovcar-8). Cells receive drug therapy the following day. After 72 hours, the culture medium is taken out, the cells are fixed with 100 μL of cold 10% Trichloroacetic acid (TCA), incubated on ice for 30 minutes, and stained with 0.4% sulforhodamine B (SRB). The data are examined using the Graphpad Prism 4 program. A four-parameter Hill equation is fitted using non-linear regression. For drug combination studies, cells are exposed simultaneously to a range of NSC 241240 concentrations along with a fixed concentration of BH3 mimics, which is predicted from single agent studies to result in a 5% growth inhibition: ABT-737, 1 μM in Ovcar-8, Ovcar-3 and Igrov-1, 2 μM in Ovcar-4 and Ovsaho and 6 μM in Cov-362; ABT-199, 1 μM in Ovcar-4, 2 μM in Ovcar-3, Igrov-1, Cov-362 and Ovsaho and 3 μM in Ovcar-8; WEHI-539, 0.2 μM in Igrov-1, 0.3 μM in Ovcar-8, 1 μM in Ovcar-3 and Ovsaho, 3.1 μM in Cov-362 and 5 μM in Ovcar-4. SRB staining is used to determine the number of living cells. It calculates a combination index (CI)[2]. | ||
| Animal Protocol |
|
||
| References |
[1]. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013 Jun;9(6):390-7. [2]. Antagonism of Bcl-XL is necessary for synergy between NSC 241240 and BH3 mimetics in ovarian cancer cells. J Ovarian Res. 2016 Apr 14;9:25. |
||
| Additional Infomation | Background: BH3 mimetics are a class of drugs that antagonize the Bcl-2 family of apoptosis inhibitors. We have previously shown that these compounds can potentiate the activity of carboplatin against several ovarian cancer cell lines. However, recent clinical studies have highlighted that BH3 mimetics which antagonise Bcl-XL are associated with significant thrombocytopenia. This has led to the development of ABT-199 which specifically inhibits Bcl-2. Unfortunately, Bcl-XL appears to be more frequently deregulated in ovarian cancer than Bcl-2. We therefore compared the ability of ABT-199, and the Bcl-XL selective compound WEHI-539, to potentiate the activity of carboplatin in ovarian cancer cell lines. Methods: WEHI-539, ABT-737 and ABT-199 were tested in combination with carboplatin using a panel of 6 ovarian cancer cell lines. The activity of the drugs was evaluated using cell growth assays, staining with trypan bue and measurement of apoptosis by measuring caspase 3/7 activity, PARP cleavage and annexin-V/propidium iodide staining. Results: We found that WEHI-539 and ABT-737, but not ABT-199, were synergistic with carboplatin in cell growth assays and potentiated cell death when assessed by trypan blue staining. Furthermore, WEHI-539 and ABT-737 augmented carboplatin induced caspase 3/7 activity, PARP cleavage and annexin V labelling, but ABT-199 failed to do so. Conclusions: These observations suggest that compounds which target Bcl-XL are necessary if BH3 mimetics are to be successfully used to treat patients with ovarian cancer and this highlights the need to develop strategies to minimize thrombocytopenia induced by such compounds.[2] |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.03 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.03 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6124 mL | 8.0622 mL | 16.1244 mL | |
| 5 mM | 0.3225 mL | 1.6124 mL | 3.2249 mL | |
| 10 mM | 0.1612 mL | 0.8062 mL | 1.6124 mL |