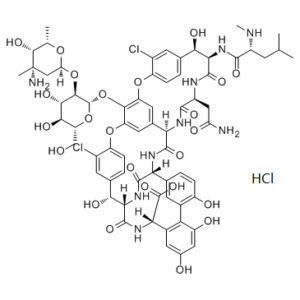
Vancomycin HCl is the hydrochloride of vancomycin that is a narrow-spectrum glycopeptide antibacterial drug used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also recommended by mouth as a treatment for severe Clostridium difficile colitis. When taken by mouth it is very poorly absorbed. Vancomycin acts by inhibiting proper cell wall synthesis in Gram-positive bacteria. Due to the different mechanism by which Gram-negative bacteria produce their cell walls and the various factors related to entering the outer membrane of Gram-negative organisms, vancomycin is not active against them (except some nongonococcal species of Neisseria).
Physicochemical Properties
| Molecular Formula | C66H75CL2N9O24.HCL |
| Molecular Weight | 1485.71 |
| Exact Mass | 1483.406 |
| Elemental Analysis | C, 53.36; H, 5.16; Cl, 7.16; N, 8.48; O, 25.84 |
| CAS # | 1404-93-9 |
| Related CAS # | Vancomycin;1404-90-6; 1404-93-9 (HCl); 123409-00-7 (HCl hydrate) |
| PubChem CID | 6420023 |
| Appearance | White to off-white solid powder. |
| Density | 1.7±0.1 g/cm3 |
| Melting Point | >190°C (dec.) |
| Flash Point | 87℃ |
| Index of Refraction | 1.735 |
| LogP | -1.44 |
| Hydrogen Bond Donor Count | 20 |
| Hydrogen Bond Acceptor Count | 26 |
| Rotatable Bond Count | 13 |
| Heavy Atom Count | 102 |
| Complexity | 2960 |
| Defined Atom Stereocenter Count | 18 |
| SMILES | C[C@@H]1O[C@@]([H])(O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2OC3=C(OC4=C(Cl)C=C([C@@H](O)[C@](NC([C@]5([H])NC6=O)=O)(C(N[C@]([H])(C(O)=O)C7=CC(O)=CC(O)=C7C8=C(O)C=CC5=C8)=O)[H])C=C4)C=C([C@@]6([H])NC([C@H](CC(N)=O)NC9=O)=O)C=C3OC%10=CC=C([C@@H](O)[C@H]9NC([C@H](NC)C(C)(C)C)=O)C=C%10Cl)C[C@](C)(N)[C@@H]1O.[H]Cl |
| InChi Key | LCTORFDMHNKUSG-XTTLPDOESA-N |
| InChi Code | InChI=1S/C66H75Cl2N9O24.ClH/c1-23(2)12-34(71-5)58(88)76-49-51(83)26-7-10-38(32(67)14-26)97-40-16-28-17-41(55(40)101-65-56(54(86)53(85)42(22-78)99-65)100-44-21-66(4,70)57(87)24(3)96-44)98-39-11-8-27(15-33(39)68)52(84)50-63(93)75-48(64(94)95)31-18-29(79)19-37(81)45(31)30-13-25(6-9-36(30)80)46(60(90)77-50)74-61(91)47(28)73-59(89)35(20-43(69)82)72-62(49)92;/h6-11,13-19,23-24,34-35,42,44,46-54,56-57,65,71,78-81,83-87H,12,20-22,70H2,1-5H3,(H2,69,82)(H,72,92)(H,73,89)(H,74,91)(H,75,93)(H,76,88)(H,77,90)(H,94,95);1H/t24-,34+,35-,42+,44-,46+,47+,48-,49+,50-,51+,52+,53+,54-,56+,57+,65-,66-;/m0./s1 |
| Chemical Name | (1S,2R,18R,19R,22S,25R,28R,40S)- 48- {[(2S,3R,4S,5S,6R)- 3- {[(2S,4S,5S,6S)- 4- amino- 5- hydroxy- 4,6- dimethyloxan- 2- yl]oxy}- 4,5- dihydroxy- 6- (hydroxymethyl)oxan- 2- yl]oxy}- 22- (carbamoylmethyl)- 5,15- dichloro- 2,18,32,35,37- pentahydroxy- 19- [(2R)- 4- methyl- 2- (methylamino)pentanamido]- 20,23,26,42,44- pentaoxo- 7,13- dioxa- 21,24,27,41,43- pentaazaoctacyclo[26.14.2.23,6.214,17.18,12.129,33.010,25.034,39]pentaconta- 3,5,8(48),9,11,14,16,29(45),30,32,34,36,38,46,49- pentadecaene- 40- carboxylic acid hydrochloride |
| Synonyms | Vancomycin HCl; Vanco-saar; Vancocin; Vancocin HCl; Vancocine. |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bacterial cell wall synthesis | ||
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay | Vancomycin is a unique glycopeptide structurally unrelated to any currently available antibiotic. It also has a unique mode of action inhibiting the second stage of cell wall synthesis of susceptible bacteria. There is also evidence that vancomycin alters the permeability of the cell membrane and selectively inhibits ribonucleic acid synthesis. Induction of bacterial L-phase variants from susceptible organisms with vancomycin is extremely difficult, and such variants are unstable. Stable L-phase variants induced by other agents are susceptible to vancomycin. Vancomycin is active against a large number of species of Gram-positive bacteria, such as Staphylococcus aureus (including methicillin-resistant strains), Staph. epidermidis (including multiple-resistant strains), Streptococcus pneumoniae (including multiple-resistant strains), Str. pyogenes, Str. agalactiae, Str. bovis, Str. mutans, viridans streptococci, enterococci, Clostridium species, diphtheroids, Listeria monocytogenes, Actinomyces species and Lactobacillus species. There has been no increase in resistance to vancomycin during the past three decades. Enhancement of antimicrobial activity has been demonstrated with the combination of vancomycin and an aminoglycoside against Staph. aureus, Str. bovis, enterococci and viridans streptococci. The combination of vancomycin and rifampicin are antagonistic to most strains of Staph. aureus, though indifference and occasionally synergism have been shown, but is synergistic against strains of Staph. epidermidis. It shows indifference against enterococci. Vancomycin and fusidic acid are indifferent against Staph. aureus [2]. | ||
| Cell Assay | C. difficile toxin assay. C. difficile toxins A and B were detected using a modified protocol for the Tech Lab Toxin A/B II ELISA kit. Each stool sample was weighed and the amount of diluent per sample was normalized to provide the same stool mass-to-diluent ratio for each sample. The diluent-sample mixtures were homogenized by grinding and vortexing, and 1:10, 1:100, and 1:1,000 serial dilutions were made of the sample. A total of 150 μl of the 1:1,000 dilution of each sample was added to a precoated well provided in the kit. A negative control consisted of 150 μl of diluent, and a positive control consisted of 135 μl of diluent plus 3 drops of the positive control toxin A-B mixture provided in the kit. One drop of conjugate was added to each well, and the plate was incubated at 37°C for 50 min. Each well was washed three times with 150 μl of a 1× dilution of the wash buffer provided in the kit. Two drops of substrate were added to each well. After 10 min, 1 drop of stop solution was added to each well. The plate was allowed to sit for 2 min before being read in an ELISA reader [3]. | ||
| Animal Protocol |
|
||
| References |
[1]. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006 Jan 1;42 Suppl 1:S35-9. [2]. Mode of action and in-vitro activity of vancomycin. J Antimicrob Chemother. 1984 Dec;14 Suppl D:7-18. [3]. Vancomycin treatment's association with delayed intestinal tissue injury, clostridial overgrowth, and recurrence of Clostridium difficile infection in mice. Antimicrob Agents Chemother. 2013 Feb;57(2):689-96. |
||
| Additional Infomation |
Vancomycin hydrochloride is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of certain bacterial infections, such as infections caused by Clostridium difficile and Staphylococcus aureus. Clostridium difficile and Staphylococcus aureus are bacteria that can cause opportunistic infections (OIs) of HIV. An OI is an infection that occurs more frequently or is more severe in people with weakened immune systems—such as people with HIV—than in people with healthy immune systems. Vancomycin Hydrochloride is the hydrochloride salt of vancomycin, a branched tricyclic glycosylated peptide with bactericidal activity against most organisms and bacteriostatic effect on enterococci. At a site different from that of penicillins and cephalosporins, vancomycin binds tightly to the D-alanyl-D-alanine portion of cell wall precursors, thereby interfering with bacterial cell wall synthesis. This leads to activation of bacterial autolysins that destroy the cell wall by lysis. Vancomycin may also alter the permeability of bacterial cytoplasmic membranes and may selectively inhibit RNA synthesis. Antibacterial obtained from Streptomyces orientalis. It is a glycopeptide related to RISTOCETIN that inhibits bacterial cell wall assembly and is toxic to kidneys and the inner ear. See also: Vancomycin (has active moiety). |
Solubility Data
| Solubility (In Vitro) | H2O : ~33.33 mg/mL (~22.43 mM ) DMSO : ~24 mg/mL (~16.15 mM ) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (1.40 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (1.40 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (1.40 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (1.40 mM) Solubility in Formulation 5: 130 mg/mL (87.50 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.6731 mL | 3.3654 mL | 6.7308 mL | |
| 5 mM | 0.1346 mL | 0.6731 mL | 1.3462 mL | |
| 10 mM | 0.0673 mL | 0.3365 mL | 0.6731 mL |