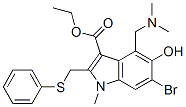
Umifenovir (trade name Arbidol) is a broad-spectrum antiviral drug that inhibits viral fusion, and has been approved for use in Russia and China for the treatment of influenza and other respiratory viral infections.
Physicochemical Properties
| Molecular Formula | C22H25BRN2O3S |
| Molecular Weight | 477.4 |
| Exact Mass | 476.076 |
| CAS # | 131707-25-0 |
| Related CAS # | Umifenovir hydrochloride;131707-23-8 |
| PubChem CID | 131411 |
| Appearance | Off-white to light yellow solid powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 591.8±50.0 °C at 760 mmHg |
| Flash Point | 311.7±30.1 °C |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.620 |
| LogP | 4.64 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Heavy Atom Count | 29 |
| Complexity | 546 |
| Defined Atom Stereocenter Count | 0 |
| InChi Key | KCFYEAOKVJSACF-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C22H25BrN2O3S/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3/h6-11,26H,5,12-13H2,1-4H3 |
| Chemical Name | ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate |
| Synonyms | Arbidol; umifenovir; |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture and light. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ln Vitro | Umifenovir has been shown to possess strong and extensive antiviral properties against a wide range of viruses, such as the influenza A, B, and C viruses; respiratory syncytial virus; SARS-CoV; adenovirus; parainfluenza type 5; rhinovirus 14; coxsackievirus B5; hantavirus; chikungunya virus; hepatitis B virus; and hepatitis C virus [1]. |
| ln Vivo | Mice with influenza who are given umifenovir orally at doses of 25 and 45 mg/ml have been shown to survive [3]. |
| Animal Protocol |
Animal/Disease Models: balb/c (Bagg ALBino) mouse (6-8 weeks old), mice were inoculated intranasally (in) with 2 times 50% mouse lethal dose (MLD50) of A/Guangdong/GIRD07/09 (H1N1) (104.5TCID50/ mL)), the volume is 20mL[3]. Doses: 1.25 mg/mL (25 mg/kg/day) and 2.25 mg/mL (45 mg/kg/day) one day before infection and 3 days after infection (dpi), total volume 500mL. Oral administration Experimental Results: Compared with the virus group, 45mg/mL can improve the survival rate and inhibit weight loss, and 25mg/mL and 45mg/mL can inhibit the increase in the lung index of mice. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Umifenovir is rapidly absorbed following oral administration, with an estimated Tmax between 0.65-1.8 hours. The Cmax has been estimated as 415 - 467 ng/mL and appears to increase linearly with dose, and the AUC0-inf following oral administration has been estimated to be approximately 2200 ng/mL/h. The major route of elimination is via the feces. Approximately 40% of an ingested dose is excreted unchanged, of which 38.9% is excreted in the bile and 0.12% excreted through the kidneys. The total recovery of parent drug and metabolites in the urine accounts for less than 1% of an ingested dose. Data regarding the volume of distribution of umifenovir are currently unavailable. In a study involving healthy male Chinese volunteers, the oral clearance of umifenovir was found to be 99 ± 34 L/h. Metabolism / Metabolites Umifenovir is highly metabolized in the body, primarily in hepatic and intestinal microsomess, with approximately 33 metabolites having been observed in human plasma, urine, and feces. The principal phase I metabolic pathways include sulfoxidation, N-demethylation, and hydroxylation, followed by phase II sulfate and glucuronide conjugation. In the urine, the major metabolites were sulfate and glucuronide conjugates, while the major species in the feces was unchanged parent drug (~40%) and the M10 metabolite (~3.0%). In the plasma, the principal metabolites are M6-1, M5, and M8 - of these, M6-1 appears of most importance given its high plasma exposure and long elimination half-life (~25h), making it a potentially important player in the safety and efficacy of umifenovir. Enzymes involved in the metabolism of umifenovir include members of the cytochrome P450 family (primarily CYP3A4), flavin-containing monooxygenase (FMO) family, and UDP-glucuronosyltransferase (UGT) family (specifically UGT1A9 and UGT2B7). Arbidol has known human metabolites that include (2S,3S,4S,5R)-6-[6-bromo-4-[(dimethylamino)methyl]-3-ethoxycarbonyl-1-methyl-2-(phenylsulfanylmethyl)indol-5-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid. Biological Half-Life The half-life of umifenovir following oral administration has been estimated to be between 17-21 hours. Serum half-lives of the M5, M6-1, and M8 metabolites were found to be 26.3 ± 5.9, 25.0 ± 5.4, and 25.7 ± 8.8, respectively. |
| Toxicity/Toxicokinetics |
Protein Binding Data regarding protein-binding of umifenovir are currently unavailable. |
| References |
[1]. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014 Jul;107:84-94. doi: 10.1016/j.antiviral.2014.04.006. Epub 2014 Apr 24. [2]. Antiviral Activity of Umifenovir In Vitro against a Broad Spectrum of Coronaviruses, Including the Novel SARS-CoV-2 Virus. Viruses. 2021 Aug 23;13(8):1665. [3]. Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret). Biomed Pharmacother. 2017 Jul;91:393-401. |
| Additional Infomation |
Pharmacodynamics Umifenovir exerts its antiviral effects via both direct-acting virucidal activity and by inhibiting one (or several) stage(s) of the viral life cycle. Its broad-spectrum of activity covers both enveloped and non-enveloped RNA and DNA viruses. It is relatively well-tolerated and possesses a large therapeutic window - weight-based doses up to 100-fold greater than those used in humans failed to produce any pathological changes in test animals. Umifenovir does not appear to result in significant viral resistance. Instances of umifenovir-resistant influenza virus demonstrated a single mutation in the HA2 subunit of influenza hemagglutinin, suggesting resistance is conferred by prevention of umifenovir’s activity related to membrane fusion. The mechanism through which other viruses may become resistant to umifenovir requires further study. |
Solubility Data
| Solubility (In Vitro) | DMSO : ~50 mg/mL (~104.73 mM) |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0947 mL | 10.4734 mL | 20.9468 mL | |
| 5 mM | 0.4189 mL | 2.0947 mL | 4.1894 mL | |
| 10 mM | 0.2095 mL | 1.0473 mL | 2.0947 mL |