UF010 (UF-010; UF 010) is a novel, potent and selective class I HDAC inhibitor with potential anticancer activities. HDAC isoforms (HDAC1, HDAC2, HDAC3, HDAC8, HDAC6, and HDAC10) are inhibited with IC50 values of 0.5 nM, 0.1 nM, 0.06 nM, 1.5 nM, 9.1 nM, and 15.3 nM, in that order. The use of histone deacetylase inhibitors (HDACi) as clinical anticancer therapies has great therapeutic promise. Yet, the HDACi that are currently on the market have unfavorable pharmacological characteristics, poor isoform selectivity, and off-target activity. For HDACi to get around these restrictions, new chemotypes are required. UF010 is a competitive inhibitor that binds to HDAC in a fast-on, slow-off manner. UF010 inhibits class I HDAC to prevent the growth of cancer cells. Global alterations in gene expression and protein acetylation result from this, which simultaneously inhibits multiple oncogenic pathways and activates tumor suppressor pathways. The UF010 class of compounds is supported in its preclinical development for potential therapeutic applications by its isotype selectivity and intriguing biological activities in suppressing tumor cell proliferation.
Physicochemical Properties
| Molecular Formula | C11H15BRN2O | |
| Molecular Weight | 271.16 | |
| Exact Mass | 270.036 | |
| Elemental Analysis | C, 48.72; H, 5.58; Br, 29.47; N, 10.33; O, 5.90 | |
| CAS # | 537672-41-6 | |
| Related CAS # |
|
|
| PubChem CID | 4596836 | |
| Appearance | White to off-white crystalline solid | |
| Density | 1.3±0.1 g/cm3 | |
| Boiling Point | 326.7±34.0 °C at 760 mmHg | |
| Flash Point | 151.4±25.7 °C | |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C | |
| Index of Refraction | 1.550 | |
| LogP | 3.46 | |
| Hydrogen Bond Donor Count | 2 | |
| Hydrogen Bond Acceptor Count | 2 | |
| Rotatable Bond Count | 5 | |
| Heavy Atom Count | 15 | |
| Complexity | 191 | |
| Defined Atom Stereocenter Count | 0 | |
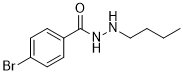
| SMILES | BrC1C([H])=C([H])C(=C([H])C=1[H])C(N([H])N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])=O |
|
| InChi Key | BVQCFCYPFJOOAV-UHFFFAOYSA-N | |
| InChi Code | InChI=1S/C11H15BrN2O/c1-2-3-8-13-14-11(15)9-4-6-10(12)7-5-9/h4-7,13H,2-3,8H2,1H3,(H,14,15) | |
| Chemical Name | 4-bromo-N'-butylbenzohydrazide | |
| Synonyms |
|
|
| HS Tariff Code | 2934.99.9001 | |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | HDAC3 (IC50 = 0.06 μM); HDAC2 (IC50 = 0.1 μM); HDAC1 (IC50 = 0.5 μM); HDAC8 (IC50 = 9.1 μM); HDAC10 (IC50 = 15.3 μM); HDAC11 (IC50 = 44.5 μM) |
| ln Vitro | UF010 primarily inhibits the G1/S transition with an increased G1 cell population and a decreased cell population in the S phase in a dose-dependent manner in cell-cycle analysis using MDA-MB-231 cells. In cell culture medium containing 10% fetal bovine serum, UF010 has a half-life of 15.8 hours[1]. |
| Cell Assay | For six hours, HCT116 and A549 cells are exposed to either DMSO or etoposide (10 μM). One hour prior to cell lysis, TSA (0.2 μM), MS-275, and UF010 (2 μM) are added. Western blotting is performed on the whole cell lysates using antibodies to the specified proteins. It is found that PCNA is a loading control. |
| References |
[1]. Chem Biol . 2015 Feb 19;22(2):273-84. |
Solubility Data
| Solubility (In Vitro) |
|
|||
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.22 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.22 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (9.22 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6879 mL | 18.4393 mL | 36.8786 mL | |
| 5 mM | 0.7376 mL | 3.6879 mL | 7.3757 mL | |
| 10 mM | 0.3688 mL | 1.8439 mL | 3.6879 mL |