Physicochemical Properties
| Molecular Formula | C40H48NO11P |
| Molecular Weight | 749.78299331665 |
| Exact Mass | 749.296 |
| CAS # | 2734877-51-9 |
| PubChem CID | 171346274 |
| Appearance | Typically exists as solid at room temperature |
| LogP | 7.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 22 |
| Heavy Atom Count | 53 |
| Complexity | 1090 |
| Defined Atom Stereocenter Count | 0 |
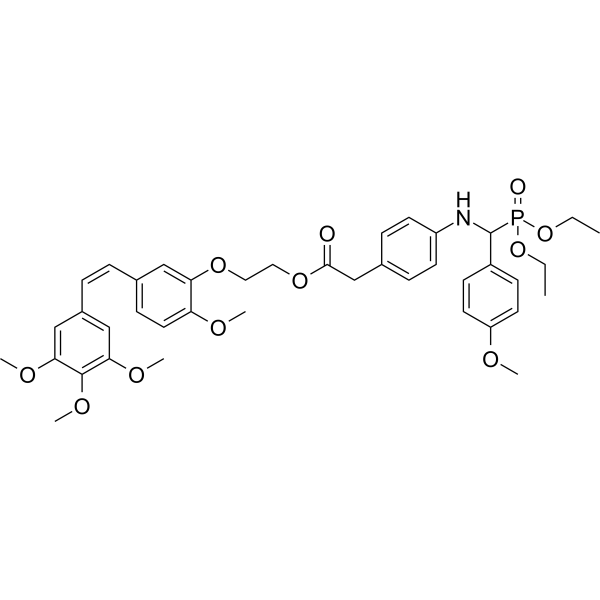
| SMILES | CCOP(=O)(C(C1=CC=C(C=C1)OC)NC2=CC=C(C=C2)CC(=O)OCCOC3=C(C=CC(=C3)/C=C\C4=CC(=C(C(=C4)OC)OC)OC)OC)OCC |
| InChi Key | DMYVJNQAJVBXBM-KHPPLWFESA-N |
| InChi Code | InChI=1S/C40H48NO11P/c1-8-51-53(43,52-9-2)40(31-15-19-33(44-3)20-16-31)41-32-17-12-29(13-18-32)27-38(42)50-23-22-49-35-24-28(14-21-34(35)45-4)10-11-30-25-36(46-5)39(48-7)37(26-30)47-6/h10-21,24-26,40-41H,8-9,22-23,27H2,1-7H3/b11-10- |
| Chemical Name | 2-[2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenoxy]ethyl 2-[4-[[diethoxyphosphoryl-(4-methoxyphenyl)methyl]amino]phenyl]acetate |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IC50: 0.36 μM (HepG-2), 0.31 μM (HT29), 0.19 μM (A549), 0.42 μM (MGC-803), 10.45 μM (LO2 cells); 0.32 μM (SK-OV-3), 0.39 μM (SK-OV-3/CDDP), 0.27 μM (MCF-7), 0.25 μM (MCF-7/DOX); 24.95 μM (MMP-2), 31.60 μM (MMP-3), 22.37 μM (MMP-9)[1]. |
| ln Vitro | For HepG-2, HT29, A549, MGC-803, and LO2 cells, tubulin/MMP-IN-2 (Compound 9e) (0.01–20 μM; 24 h) exhibits activity with IC50 values of 0.36 μM, 0.31 μM, 0.19 μM, 0.42 μM, and 10.45 μM, respectively[1]. For SK-OV-3, SK-OV-3/CDDP, MCF-7, and MCF-7/DOX cells, tubulin/MMP-IN-2 exhibits anti-proliferative activity with IC50 values of 0.32 μM, 0.39 μM, 0.27 μM, and 0.25 μM, respectively[1]. MMP-2, MMP-3, and MMP-9 are all inhibited by tubulin/MMP-IN-2, with IC50 values of 24.95 μM, 31.60 μM, and 22.37 μM, respectively[1]. Strongly inhibiting tubulin polymerization and inducing cell death and cell cycle arrest in the G2/M stage, tubulin/MMP-IN-2 (2.5, 5 Mm; 24 h) notably exhibited reduction of cell migration against A549 cells in vitro[1]. Apoptosis can be induced by tubulin/MMP-IN-2 (2.5, 5 μm; 24 h) through the mitochondria-dependent apoptosis pathway[1]. ER stress can also be caused by tubulin/MMP-IN-2 (2.5, 5 μm; 24 h), which manifests as an up-regulation of protein expression (CHOP, p-elF2a, and p-PERK)[1]. |
| ln Vivo | In A549 xenograft models, tubulin/MMP-IN-2 (15, 30 mg/kg; every two days for three weeks) exhibits strong in vivo anticancer activity without producing evident systemic toxicity[1]. |
| Cell Assay |
Cell Cytotoxicity Assay[1] Cell Types: HepG-2, HT-29, A549, MGC-803, SK-OV-3, MCF-7, SK-OV-3/CDDP, MCF-7/DOX and normal liver cells LO2 Tested Concentrations: 0.01-20 μM Incubation Duration: 72 h Experimental Results: demonstrated the most potent activity against various human cancer cells as well as multidrug-resistant tumor cells (A549/CDDP and MCF-7 /DOX) and also demonstrated Dramatically lower cytotoxic activity toward human normal liver cells LO2. Apoptosis Analysis[1] Cell Types: A549 cells Tested Concentrations: 2.5, 5 μM Incubation Duration: 24 h Experimental Results: Dramatically induced apoptosis after 24 h. Cell Cycle Analysis [1] Cell Types: A549 cells Tested Concentrations: 2.5, 5 μM Incubation Duration: 24 h Experimental Results: Induced a concentration-dependent G2/M stage arrest of A549 cells. Immunofluorescence[1] Cell Types: A549 cells Tested Concentrations: 2.5, 5 μM Incubation Duration: 24 h Experimental Results: Remarkably induced changes in cell morphology, such as loss of membrane protrusions, disrupted microtubule organization and microtubule depolymerization, respectively. |
| Animal Protocol |
Animal/Disease Models: BALB/c nude mice[1] Doses: 15, 30 mg/kg Route of Administration: Every two days for three weeks Experimental Results: demonstrated a dose-dependent inhibitory effect on tumor growth. demonstrated no obvious histopathological changes for the main organ tissues (eg heart, liver, spleen, lung and kidney). |
| References | [1]. Xiaochao Huang, et al. Novel combretastatin A-4 derivative containing aminophosphonates as dual inhibitors of tubulin and matrix metalloproteinases for lung cancer treatment. Eur J Med Chem. 2022 Dec 15;244:114817. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3337 mL | 6.6686 mL | 13.3372 mL | |
| 5 mM | 0.2667 mL | 1.3337 mL | 2.6674 mL | |
| 10 mM | 0.1334 mL | 0.6669 mL | 1.3337 mL |