Physicochemical Properties
| Molecular Formula | C14H22N2O3 |
| Molecular Weight | 266.34 |
| Exact Mass | 274.213 |
| Elemental Analysis | C, 63.13; H, 8.33; N, 10.52; O, 18.02 |
| CAS # | 5011-34-7 |
| Related CAS # | Trimetazidine dihydrochloride;13171-25-0 |
| PubChem CID | 21109 |
| Appearance | White to off-white ointment |
| Density | 1.1±0.1 g/cm3 |
| Boiling Point | 364.0±37.0 °C at 760 mmHg |
| Melting Point | 200 - 205ºC |
| Flash Point | 174.0±26.5 °C |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.524 |
| LogP | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Heavy Atom Count | 19 |
| Complexity | 259 |
| Defined Atom Stereocenter Count | 0 |
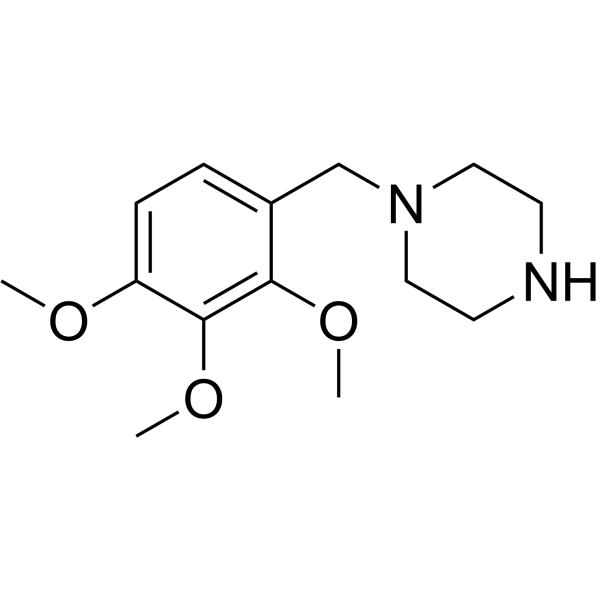
| SMILES | COC1=C(C(=C(C=C1)CN2CCNCC2)OC)OC |
| InChi Key | UHWVSEOVJBQKBE-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C14H22N2O3/c1-17-12-5-4-11(13(18-2)14(12)19-3)10-16-8-6-15-7-9-16/h4-5,15H,6-10H2,1-3H3 |
| Chemical Name | 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine |
| Synonyms | TRIMETAZIDINE; 5011-34-7; 1-(2,3,4-Trimethoxybenzyl)piperazine; 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine; 1-(2,3,4-Trimethoxy-benzyl)-piperazine; Piperazine, 1-((2,3,4-trimethoxyphenyl)methyl)-; N9A0A0R9S8; Trimetazidine (INN); |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: (1). This product requires protection from light (avoid light exposure) during transportation and storage.(2). Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | IC50: 75 nM (long chain 3-ketoyl coenzyme A thiolase)[2] β-oxidation[2] Autophagy[3] 3-hydroxyacyl-CoA dehydrogenase (HADHA)[4] |
| ln Vitro | In a dose-dependent way, trimetazidine (1–100 μM; 24 h; HUVECs) improves the viability of HUVECs that have undergone oxidative damage [1]. |
| ln Vivo | At doses of 10 and 20 mg/kg, trimetazidine (5–20 mg/kg; PO; 1 hour; Swiss albino male mice) markedly elevated seizure threshold current in the mouse ICES test [5]. |
| Enzyme Assay | Trimetazidine had no effect on myocardial oxygen consumption or cardiac work under any aerobic perfusion condition used. In hearts perfused with 5 mmol/L glucose and 0.4 mmol/L palmitate, trimetazidine decreased the rate of palmitate oxidation from 488+/-24 to 408+/-15 nmol x g dry weight(-1) x minute(-1) (P<0.05), whereas it increased rates of glucose oxidation from 1889+/-119 to 2378+/-166 nmol x g dry weight(-1) x minute(-1) (P<0.05). In hearts subjected to low-flow ischemia, trimetazidine resulted in a 210% increase in glucose oxidation rates. In both aerobic and ischemic hearts, glycolytic rates were unaltered by trimetazidine. The effects of trimetazidine on glucose oxidation were accompanied by a 37% increase in the active form of pyruvate dehydrogenase, the rate-limiting enzyme for glucose oxidation. No effect of trimetazidine was observed on glycolysis, glucose oxidation, fatty acid oxidation, or active pyruvate dehydrogenase when palmitate was substituted with 0.8 mmol/L octanoate or 1.6 mmol/L butyrate, suggesting that trimetazidine directly inhibits long-chain fatty acid oxidation. This reduction in fatty acid oxidation was accompanied by a significant decrease in the activity of the long-chain isoform of the last enzyme involved in fatty acid beta-oxidation, 3-ketoacyl coenzyme A (CoA) thiolase activity (IC(50) of 75 nmol/L). In contrast, concentrations of trimetazidine in excess of 10 and 100 micromol/L were needed to inhibit the medium- and short-chain forms of 3-ketoacyl CoA thiolase, respectively. Previous studies have shown that inhibition of fatty acid oxidation and stimulation of glucose oxidation can protect the ischemic heart. Therefore, our data suggest that the antianginal effects of trimetazidine may occur because of an inhibition of long-chain 3-ketoacyl CoA thiolase activity, which results in a reduction in fatty acid oxidation and a stimulation of glucose oxidation[3]. |
| Cell Assay |
Cell Viability Assay[1] Cell Types: Human umbilical vein endothelial cells (HUVECs) Tested Concentrations: 1 μM,10 μM,100 μM Incubation Duration: 24 hrs (hours) Experimental Results: Enhanced the viability of the injured HUVECs induced by oxidation. |
| Animal Protocol |
Animal/Disease Models: Swiss albino male mice (24-35 g)[4] Doses: 5 mg/kg, 10 mg/kg and 20 mg/kg; 10 mL/kg body weight Route of Administration: Oral administration; 1 hour Experimental Results: In 10 and 20mg/kg doses Dramatically raised the seizure-threshold current in the ICES test. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion In elderly patients, a 35 mg oral modified release tablet reaches a mean Cmax of 115 µg/L, with a Tmax of 2.0-5.0 hours, and a mean AUC0-12 of 1104 h\*µg/L. In young, healthy patients, the same dose reaches a mean Cmax of 91.2 µg/L, with a Tmax of 2.0-6.0 hours, and an AUC0-12h 720 h\*µg/L. Trimetazidine is 79-84% eliminated in the urine, with 60% as the unchanged parent compound. In a study of 4 healthy subjects, individual metabolites made up 0.01-1.4% of the dose recovered in urine. In the urine, 2-desmethyltrimetazidine made up 0-1.4% of the recovered dose, 3- and 4-desmethyltrimetazidine made up 0.039-0.071% each, N-methyltrimetazidine made up 0.015-0.11%, trimetazidine ketopiperazine made up 0.011-0.4%, N-formyltrimetazidine made up 0.035-0.42%, N-acetyltrimetazidine made up 0.016-0.19%, desmethyl trimetazidine O-sulphate made up 0.01-0.65%, and an unknown metabolite made up0.026-0.67%. The volume of distribution of trimetazidine is 4.8 L/kg. Trimetazidine clearance is strongly correlated with creatinine clearance. In eldery patients with a creatinine clearance of 72 ± 8 mL/min, trimetazidine clearance was 15.69 L/h. In young, healthy patients with a creatinine clearance of 134 ± 18 mL/min, trimetazidine clearance was 25.2 L/h. Metabolism / Metabolites Trimetazidine can be oxidized at the piperazine ring to form trimetazidine ketopiperazine. Trimetazidine can also be N-formylated, N-acetylated, or N-methylated at the piperazine ring to form N-formyltrimetazidine, N-acetyltrimetazidine, and N-methyltrimetazidine respectively. Alternatively, trimetazidine can be demethylated at the 2, 3, or 4 position of the 2,3,4-trimethoxybenzyl moiety to form 2-desmethyltrimetazidine, 3-desmethyltrimetazidine, or 4-desmethyltrimetazidine. The desmethyltrimetazidine metabolites can undergo sulfate conjugation or glucuronidation prior to elimination. Biological Half-Life In young, healthy subjects, the half life of trimetazidine is 7.81 hours. In patients over 65, the half life increases to 11.7 hours. |
| Toxicity/Toxicokinetics |
Protein Binding Trimetazidine is 15% protein bound in plasma. Trimetazidine can bind to human serum albumin. |
| References |
[1]. Protective effects of trimetazidine against vascular endothelial cell injury induced by oxidation. Journal of Geriatric Cardiology, December 2008 , Vol 5 No 4. [2]. Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs. 2014 Jun;74(9):971-80. [3]. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000 Mar 17;86(5):580-8. [4]. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015 Nov;3(11):1236-47. [5]. Trimetazidine exerts protection against increasing current electroshock seizure test in mice. Seizure. 2010 Jun;19(5):300-2. |
| Additional Infomation |
1-[(2,3,4-trimethoxyphenyl)methyl]piperazine is an aromatic amine. Trimetazidine is a piperazine derivative indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Trimetazidine has been studied as a treatment for angina pectoris since the late 1960s. Acidic conditions, caused by anaerobic metabolism and fatty acid oxidation, in response to myocardial ischemia, activate sodium-hydrogen and sodium-calcium antiport systems. The increased intracellular calcium decreases contractility. It is hypothesized that trimetazidine inhibits 3-ketoacyl coenzyme A thiolase, which decreases fatty acid oxidation but not glucose metabolism, preventing the acidic conditions that exacerbate ischemic injury. However, evidence for this mechanism is controversial. Trimetazidine is not FDA approved. However, it has been approved in France since 1978. Trimetazidine is an orally available small molecule compound with anti-ischemic, and potential immunomodulating and antineoplastic properties. Although its exact mechanism is not yet fully elucidated, it is postulated that upon administration, trimetazidine selectively inhibits long-chain 3-ketoacyl coenzyme A thiolase (LC 3-KAT), the final enzyme in the free fatty acid (FFA) beta-oxidation pathway. This stimulates glucose oxidation, which requires less oxygen usage and cellular energy than in the beta-oxidation process. This optimizes myocardial energy metabolism and cardiac functioning in an ischemic condition. In cancer cells, the inhibition of fatty acid oxidation (FAO) alters the metabolic processes needed for tumor cell function and proliferation, thereby inducing tumor cell apoptosis. In addition, inhibition of FAO may potentially block the immunosuppressive functions of myeloid-derived suppressor cells (MSDCs), which are thought to promote malignant cell proliferation and migration by inhibiting T-cell function. A vasodilator used in angina of effort or ischemic heart disease. Drug Indication Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Mechanism of Action During myocardial ischemia, anaerobic metabolism takes over, increasing levels of lactic acid. The decreased intracellular pH and increased concentration of protons activates sodium-hydrogen and sodium-calcium antiport systems, raising intracellular calcium concentrations, finally leading to decreased contractility. This injury to the myocardium raises concentrations of catecholamines, which activate hormone sensitive lipase, and increasing fatty acid concentrations in plasma. When the myocardium is repurfused, fatty acid oxidation becomes the dominant form of ATP production, maintaining an acidic pH, and further exacerbating the injury. The mechanism of action of trimetazidine is not fully understood. Trimetazidine may inhibit mitochondrial 3-ketoacyl coenzyme A thiolase, decreasing long chain fatty acid β-oxidation but not glycolysis in the myocardium. The decreased long chain fatty acid β-oxidation is compensated for by increased use of glucose, preventing a lowered myocardial pH, and further decreases in contractility. However, another study suggests that 3-ketoacyl coenzyme A thiolase may not be trimetazidine's target, and that this mechanism may be incorrect. Pharmacodynamics Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Patients should be counselled regarding the risk of use with reduced renal or hepatic function, worsening of extrapyramidal symptoms or other movement disorders, and risk of falls. |
Solubility Data
| Solubility (In Vitro) | DMSO : ≥ 125 mg/mL (469.32 mM) |
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (7.81 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (7.81 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.08 mg/mL (7.81 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7546 mL | 18.7730 mL | 37.5460 mL | |
| 5 mM | 0.7509 mL | 3.7546 mL | 7.5092 mL | |
| 10 mM | 0.3755 mL | 1.8773 mL | 3.7546 mL |