Physicochemical Properties
| Molecular Formula | C15H15CLF3N3O |
| Molecular Weight | 345.75 |
| Exact Mass | 345.085 |
| CAS # | 68694-11-1 |
| PubChem CID | 91699 |
| Appearance | Colorless crystals |
| Density | 1.3±0.1 g/cm3 |
| Boiling Point | 421.2±55.0 °C at 760 mmHg |
| Melting Point | 63.5°C |
| Flash Point | 208.6±31.5 °C |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.533 |
| LogP | 4.66 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Heavy Atom Count | 23 |
| Complexity | 406 |
| Defined Atom Stereocenter Count | 0 |
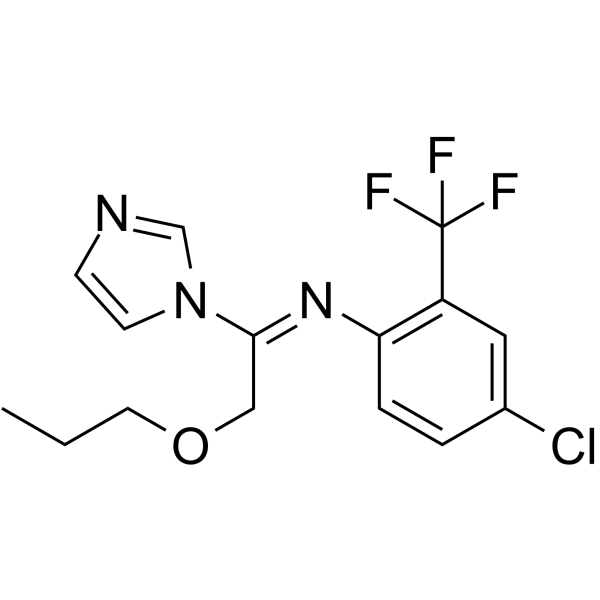
| SMILES | CCCOC/C(=N\C1=C(C=C(C=C1)Cl)C(F)(F)F)/N2C=CN=C2 |
| InChi Key | HSMVPDGQOIQYSR-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C15H15ClF3N3O/c1-2-7-23-9-14(22-6-5-20-10-22)21-13-4-3-11(16)8-12(13)15(17,18)19/h3-6,8,10H,2,7,9H2,1H3 |
| Chemical Name | N-[4-chloro-2-(trifluoromethyl)phenyl]-1-imidazol-1-yl-2-propoxyethanimine |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion Following oral treatment of rats with /a single oral dose of 10 mg/kg/ [phenyl- U-(14)C]-NF-114, no sex-related differences were observed in absorption, metabolism, distribution or excretion. Maximum concentrations of radioactivity in plasma were attained within 1 hour of dosing in both sexes. Low levels of radioactivity were detectable in all tissue, organ, and blood samples. Radioactivity in urine accounted for 69.5-74.4% of the dose and feces accounted for 21.7-21.9% of the dose. /From table/ M&F: 10 or 300 mg/kg as single oral dose: Following oral treatment of rats with [phenyl- U-(14)C]-NF-114, approximately 93.8-100.6% of the administered dose was recovered. Urine was the major route of excretion. Low levels of radioactivity were detectable in all tissue, organ, and blood samples collected 2 days (10 mg/kg group) or 4 days (300 mg/kg group) post-dose with tissue concentrations generally higher in males than females. The metabolite profile in the excreta was quantitatively and qualitatively similar between the sexes and dose groups. /From table/ Metabolism / Metabolites The metabolism of triflumizole was investigated by analyzing fecal and urine samples retained from ... previous studies: single oral administration of 10 or 300 mg/kg bw and 14 consecutive oral doses of 10 mg/kg bw per day. ... Triflumizole is extensively metabolized: less than 2% of the radiolabel recovered from urine or feces was identified as parent compound. A few differences in metabolite pattern were observed between males and females after repeated low and single high doses, but not after a single low dose. The major urinary metabolites are the sulfate conjugates of FM-8-1 and FA-1-5, each representing approximately 20% of the radiolabel recovered after the single low dose from that matrix and, respectively, approximately 11% and 20% after the high dose. In feces, FD-2-1 is a major metabolite in all dose regimens (~6-10% of the recovered radiolabel). Considerable differences between dose regimens exist with respect to other major metabolites. FM-2-1 is the major metabolite after a single oral low dose (~9% of radiolabel recovered in feces), but represents less than 2% for the other dose regimens. FA-1-1 is a major metabolite after single and repeated low dosing (~5-10%), but not after single oral high dosing (<3%), whereas FD-1-1 is the major metabolite after a single oral high dose (~16%) and a minor metabolite after single and repeated low doses (<2%). The metabolite FM-6-1 was tentatively identified by TLC co-chromatography, and the radioactive band corresponding to the authentic FM-6-1 was obscure, because of its low radioactivity. |
| Toxicity/Toxicokinetics |
Toxicity Summary IDENTIFICATION AND USE: Triflumizole is a fungicide used for the control of powdery mildew, such as Sphaerotheca fuliginea, Sphaerotheca pannosa, Erysiphe cichoracearum, and others. HUMAN EXPOSURE AND TOXICITY: A report on the results of its yearly health examination of the personnel involved in the production of triflumizole at the Takaoka plant (Japan) during the period May 1996 to May 2002 was published. No adverse health effects attributable to chemical exposure were observed. In addition, it was reported that in the period covered, no events of acute poisoning by exposure or skin and/or eye irritation were observed. Repeated or prolonged contact may cause skin sensitization. The substance may have effects on the liver and blood. This may result in liver impairment and a decrease in hemoglobin. ANIMAL STUDIES: Triflumizole is sensitizing to the skin of guinea pigs. It is mildly irritating to the eye of rabbits. An acute inhalation toxicity test for triflumizole was conducted in rats. Symptoms of toxicity included hunched posture, lethargy and chromodacryorrhoea (head and/or snout) among the majority of the animals. Signs of oral toxicity from triflumizole observed in rats included ataxia, hypotonia, ventral position, lacrimation, urinary incontinence, decreased body temperature, decreased heart rate and respiration rate, and ptosis. Triflumizole did not produce tumors in rats after 104 weeks of treatment. In developmental studies in rats no statistically significant treatment-related external, visceral, or skeletal malformations or variations were noted. In a mouse study, prenatal triflumizole exposure increases adipose depot weight and diverts mesenchymal stromal stem cells fate toward the adipocyte lineage. Triflumizole was not mutagenic in the Ames test with or without metabolic activation. ECOTOXICITY STUDIES: Using rare minnow (Gobiocypris rarus) at early-life stages, the developmental toxicity of five widely used triazole fungicides including triflumizole was rated as highly toxic. Non-Human Toxicity Values LD50 Rat oral 2230 mg/kg (Terraguard 50W) LD50 Rat (male) oral 1057 mg/kg bw LD50 Rat (female) oral 1780 mg/kg bw LC50 Rat inhalation >3.6 mg/L/4 hr For more Non-Human Toxicity Values (Complete) data for Triflumizole (6 total), please visit the HSDB record page. |
| References |
[1]. Acute toxicity of triflumizole to freshwater green algae Chlorella vulgaris. Pestic Biochem Physiol. 2019 Jul;158:135-142. |
| Additional Infomation | Triflumizole is a carboxamidine resulting from the formal condensation of the amino group of 4-chloro-2-(trifluoromethyl)aniline with the oxygen of the acetyl group of N-(propoxyacetyl)imidazole. A sterol demethylation inhibitor, it is used as a fungicide for the control of powdery mildew, scab and other diseases on a variety of crops. It has a role as an EC 1.14.13.70 (sterol 14alpha-demethylase) inhibitor and an antifungal agrochemical. It is a member of monochlorobenzenes, a member of imidazoles, a member of (trifluoromethyl)benzenes, a carboxamidine, an ether, a conazole fungicide and an imidazole fungicide. |
Solubility Data
| Solubility (In Vitro) | May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples |
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples. Injection Formulations (e.g. IP/IV/IM/SC) Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] *Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Injection Formulation 5: 2-Hydroxypropyl-β-cyclodextrin : Saline = 50 : 50 (i.e. 500 μL 2-Hydroxypropyl-β-cyclodextrin → 500 μL Saline) Injection Formulation 6: DMSO : PEG300 : castor oil : Saline = 5 : 10 : 20 : 65 (i.e. 50 μL DMSO → 100 μLPEG300 → 200 μL castor oil → 650 μL Saline) Injection Formulation 7: Ethanol : Cremophor : Saline = 10: 10 : 80 (i.e. 100 μL Ethanol → 100 μL Cremophor → 800 μL Saline) Injection Formulation 8: Dissolve in Cremophor/Ethanol (50 : 50), then diluted by Saline Injection Formulation 9: EtOH : Corn oil = 10 : 90 (i.e. 100 μL EtOH → 900 μL Corn oil) Injection Formulation 10: EtOH : PEG300:Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL EtOH → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Oral Formulations Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). Oral Formulation 3: Dissolved in PEG400 Oral Formulation 4: Suspend in 0.2% Carboxymethyl cellulose Oral Formulation 5: Dissolve in 0.25% Tween 80 and 0.5% Carboxymethyl cellulose Oral Formulation 6: Mixing with food powders Note: Please be aware that the above formulations are for reference only. InvivoChem strongly recommends customers to read literature methods/protocols carefully before determining which formulation you should use for in vivo studies, as different compounds have different solubility properties and have to be formulated differently. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8923 mL | 14.4613 mL | 28.9226 mL | |
| 5 mM | 0.5785 mL | 2.8923 mL | 5.7845 mL | |
| 10 mM | 0.2892 mL | 1.4461 mL | 2.8923 mL |