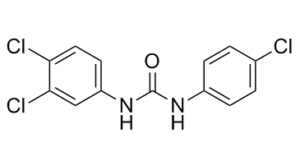
Physicochemical Properties
| Molecular Formula | C13H9CL3N2O |
| Molecular Weight | 315.5824 |
| Exact Mass | 313.978 |
| Elemental Analysis | C, 49.48; H, 2.87; Cl, 33.70; N, 8.88; O, 5.07 |
| CAS # | 101-20-2 |
| Related CAS # | Triclocarban-d4;1219799-29-7 |
| PubChem CID | 7547 |
| Appearance |
Fine white plates Fine plates Fine, white to off-white powder |
| Density | 1.4±0.1 g/cm3 |
| Boiling Point | 475.3±55.0 °C at 760 mmHg |
| Melting Point | 254-256 °C(lit.) |
| Flash Point | 241.2±31.5 °C |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.630 |
| LogP | 5.66 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Heavy Atom Count | 19 |
| Complexity | 308 |
| Defined Atom Stereocenter Count | 0 |
| SMILES | ClC1=C(C([H])=C([H])C(=C1[H])N([H])C(N([H])C1C([H])=C([H])C(=C([H])C=1[H])Cl)=O)Cl |
| InChi Key | ICUTUKXCWQYESQ-UHFFFAOYSA-N |
| InChi Code | InChI=1S/C13H9Cl3N2O/c14-8-1-3-9(4-2-8)17-13(19)18-10-5-6-11(15)12(16)7-10/h1-7H,(H2,17,18,19) |
| Chemical Name | Carbanilide, 3,4,4'-trichloro- |
| Synonyms | 3,4,4′-Trichlorocarbanilide |
| HS Tariff Code | 2934.99.9001 |
| Storage |
Powder-20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition | Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs) |
Biological Activity
| Targets | Bacterial |
| ln Vitro | Rat thymocytes exposed to 300 µM H2O2 have greater cytotoxicity when treated with triclocarban (300 nM). Triclocarban (300 nM) promotes the process of H2O2-induced cell death, which leads to an additional increase in the population of dead cells[1]. It does not itself increase the population of death cells. Triclocarban exhibits its estrogenic effects by stimulating luciferase activity in an ER reporter gene assay, encouraging MCF-7 cell proliferation, up-regulating pS2 expression, and down-regulating ERα expression in MCF-7 cells at both the mRNA and protein levels. |
| ln Vivo | Human subjects' use of soap during showering causes triclocarbaban to be absorbed significantly; its Cmax in their whole blood ranges from 23 nM to 530 nM[1]. Exposure to triclocarban during gestation does not impact a mother's ability to carry her offspring to term, but exposure to triclocarban during lactation negatively impacts the offspring's survival[3]. |
| Animal Protocol | Rats: In three experiments that limited exposure to critical growth periods—gestation, gestation and lactation, or lactation only (cross-fostering)—Sprague Dawley rats are given control, 0.2% weight/weight (w/w), or 0.5% w/w triclocarban-supplemented chow. The goal was to identify the susceptible windows of exposure for developmental consequences.[3] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion A human exposure study in a small group of subjects demonstrated that a portion of the TCC present in bar soaps is absorbed through the skin and is excreted in urine as N-glucuronides. Because they are produced and used in large quantities in various products, they are absorbed into the human body of the general population. The absorption of triclocarban during a human pharmacokinetic study was estimated at 0.6% of the 70 + or - 15 mg of triclocarban in the soap used. The triclocarban-N-glucuronide urine concentration varied considerably among the study subjects, and continuous daily use of the soap led to steady-state levels of excretion. The metabolism of (14)C-TCC (3,4,4'-trichlorocarbanilide) has been investigated in humans following oral exposure to 2.2 mumol/kg. Fecal elimination (70% of dose) was complete at the 120 hour point after administration and the urinary excretion (27% of dose) was complete after 80 hours post-administration. Urinary glucuronides appear to be valuable biomarkers of triclocarban exposure. After a pharmacokinetic study in man, radioactivity was rapidly cleared from blood after intravenous administrations of (14)C-triclocarban in propylene glycol with a blood clearance half-life measured to be 8.6 hours. /MILK/ ... Sprague Dawley rats were provided control, 0.2% weight/weight (w/w), or 0.5% w/w TCC-supplemented chow through a series of 3 experiments that limited exposure to critical growth periods: gestation, gestation and lactation, or lactation only (cross-fostering) to determine the susceptible windows of exposure for developmental consequences. ... The average concentration of TCC in the milk was almost 4 times that of the corresponding maternal serum levels. The results demonstrate that gestational TCC exposure does not affect the ability of dams to carry offspring to term but TCC exposure during lactation has adverse consequences on the survival of offspring although the mechanism of reduced survival is currently unknown. This information highlights the importance of evaluating the safety of TCC application in personal care products and the impacts during early life exposure. The antibacterial triclocarban (TCC) concentrates in the cellular fraction of blood. Consequently, plasma levels are at least two-fold lower than the TCC amount present in blood. Utilizing whole blood sampling, a low but significant absorption of TCC from soap during showering is demonstrated for a small group of human subjects. The route and rate of excretion by rats of the germicide (14)C-triclocarban formerly called trichlorocarbanilide, given by parenteral injection has been investigated. Blood levels based on radioactivity and by chemical determination after parenteral injection have been compared with those obtained after topical application of (14)C-triclocarban in soaps and in dimethylformamide (DMF) through occluded rat skin has been studied. Other soaps and a hand cleanser containing (14)C-triclocarban have been applied to rat skin without occlusion and the effects of duration of contact, concentration and the use of a solubilizer have been investigated. In humans, absorption of triclocarban through skin after bathing daily for 28 days has been investigated by chemical analysis of blood and urine. The data show that elimination by the rat is rapid and complete principally via the feces. Blood levels after parenteral injection are low and comparison of the radioactivity and chemical determinations suggest rapid metabolism of the Triclocarban. After application to the skin, blood levels based on (14)C-triclocarban are very low. Absorption of (14)C-triclocarban through occluded rat skin was greater from DMF than from soaps. With non-occluded rat skin, absorption from soaps was less and was dependent on concentration but independent of duration of contact. The use of a solubilizer did not increase absorption through skin. No measurable triclocarban (less than 25 ppb) was present in blood and urine samples of volunteers during or shortly after a 28-day intensive bathing regimen. The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. Fecal elimination (70% of dose) was complete 120 hr after dosing and the urinary excretion (27% of dose) was completed in 80 hr. The maximum plasma level occurred 2.8 hr after dosing and was 3.7 nmol-equivalents of TCC per g of plasma (approximately 1.2 ppm). Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ... For more Absorption, Distribution and Excretion (Complete) data for Triclocarban (10 total), please visit the HSDB record page. Metabolism / Metabolites Blood levels after parenteral injection are low and comparison of the radioactivity and chemical determinations suggest rapid metabolism of the Triclocarban. Human metabolism of TCC involves direct glucuronidation to form N- and N'- glucuronides as well as ring hydroxylation to 2'-hydroxy-TCC and 6-hydroxy-TCC, which are further metabolized to sulfate and glucuronide conjugates. In human subjects given a single oral dose of TCC, 27% of the dose was excreted in the urine within 80 hours. About 70% of the administered dose was excreted in the feces within 5 days. The major urinary metabolites were N-glucuronides (average levels, 30 ng/mL) and a major plasma metabolite was the sulfate conjugate of 2'-OH-TCC (levels ranged from 0-20 ng/mL. The maximum plasma level occurred 2.8 hr after dosing and was 3.7 nmol-equivalents of TCC per g of plasma (approximately 1.2 ppm). Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half-life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. Fecal elimination (70% of dose) was complete 120 hr after dosing and the urinary excretion (27% of dose) was completed in 80 hr. ... Biotransformation of TCC was rapid but did not appear to involve splitting of the basic TCC structure. The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ... Plant uptake and metabolism of emerging organic contaminants, such as personal-care products, pose potential risks to human health. In this study, jalapeno pepper (Capsicum annuum) plants cultured in hydroponic media were exposed to both (14)C-labeled and unlabeled triclocarban (TCC) to investigate the accumulation, distribution, and metabolism of TCC following plant uptake. The results revealed that TCC was detected in all plant tissues; after 12 weeks, the TCC concentrations in root, stem, leaf, and fruit tissues were 19.74 +/- 2.26, 0.26 +/- 0.04, 0.11 +/- 0.01, and 0.03 +/- 0.01 mg/kg dry weight, respectively. More importantly, a substantial portion of the TCC taken up by plants was metabolized, especially in the stems, leaves, and fruits. Hydroxylated TCC (e.g., 2'-OH TCC and 6-OH TCC) and glycosylated OH-TCC were the main phase I and phase II metabolites in plant tissues, respectively. Bound (or nonextractable) residues of TCC accounted for approximately 44.6, 85.6, 69.0, and 47.5% of all TCC species that accumulated in roots, stems, leaves, and fruits, respectively. The concentrations of TCC metabolites were more than 20 times greater than the concentrations of TCC in the above-ground tissues of the jalapeno pepper plants after 12 weeks; crucially, approximately 95.6% of the TCC was present as metabolites in the fruits. Consequently, human exposure to TCC through the consumption of pepper fruits is expected to be substantially higher when phytometabolism is considered. Previous studies of triclocarban suggest that its biotransformation could yield reactive metabolites that form protein adducts. Since the skin is the major route of triclocarban exposure, present work examined this possibility in cultured human keratinocytes. The results provide evidence for considerable biotransformation and protein adduct formation when cytochrome P450 activity is induced in the cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin, a model Ah receptor ligand. Since detecting low adduct levels in cells and tissues is difficult, we utilized the novel approach of accelerator mass spectrometry for this purpose. Exploiting the sensitivity of the method, we demonstrated that a substantial portion of triclocarban forms adducts with keratinocyte protein under the P450 inducing conditions employed. ... After repeated oral administration of 3,4,4'-trichlorocarbanilide (TCC) ... the biliary metabolites ... were isolated and identified. The major TCC biliary metabolite was found to be 2'-hydroxy-TCC. This compound was isolated mainly from the nonconjugated and the glucuronide fractions. Other metabolites present in substantial quantities were 6-hydroxy-TCC and 2',6-dihydroxy-TCC mainly as glucuronides and 3'-hydroxy-TCC mainly as the sulfate conjugate. Small amounts of 3',6-dihydroxy-TCC were isolated from each of the fractions. No unchanged TCC was found in the bile. Only traces of other metabolites were found, and no N-hydroxylated products were observed. ... For more Metabolism/Metabolites (Complete) data for Triclocarban (7 total), please visit the HSDB record page. Biological Half-Life 10 hours The metabolism and disposition of (14)C-TCC (3,4,4'-trichlorocarbanilide) have been evaluated in humans following oral exposure to 2.2 umol/kg body wt. ... The major plasma metabolites were N- and N'-glucuronides of TCC which were eliminated with half life approximately 2 hr to the urine and 2'-hydroxy-TCC sulfate and 6-hydroxy-TCC sulfate (the o-hydroxy-TCC sulfates) which were removed with half life approximately 20 hr (presumably into the bile). ... ... Radioactivity was rapidly cleared from blood after intravenous administrations of (14)C-triclocarban in propylene glycol with a blood clearance half-life of 8.6 hours. About 54% of the dose was excreted in the feces and 21% of the dose in the urine with a urinary elimination half-life of ten hours. ... |
| Toxicity/Toxicokinetics |
Toxicity Summary IDENTIFICATION AND USE: Triclocarban (TCC) is a solid. It has anti-microbial activity and is used globally in a wide range of personal cleansing products that include deodorant soaps, detergents, cleansing lotions, and wipes. In North America, TCC is used exclusively as an antimicrobial and preservative in bar and liquid soaps and body washes. HUMAN STUDIES: Studies in humans indicate no evidence of skin sensitization or severe irritation. Petrolatum (0.1mL) containing 0, 1, 3, 6, and 9% TCC was applied continuously for 21 days on ten males. Minimal skin irritation was observed with the 9% concentration. There was no evidence of skin sensitization in volunteers tested with TCC (1.5% and 10%). The potential impact of exposure to TCC on fetal abnormalities was studied in 39 pregnant women diagnosed with fetal or post-birth abnormalities. Significantly increased levels of TCC were detected in maternal sera from mothers with abnormal births. TCC was also studied for its endocrine-disrupting effects. TCC exerted estrogenic activities by inducing luciferase activities in an ER reporter gene assay, promoting the proliferation of the MCF-7 cells, up-regulating the expression of pS2 and down-regulating ERalpha expression at both the mRNA and protein levels in the MCF-7 cells. In prostate cancer-derived LNCaP and C4-2B cells, TCC potentiated androgen actions via androgen receptor-dependent actions. ANIMAL STUDIES: TCC was non-irritating to guinea pig and rabbit skin. TCC did not cause skin sensitization in guinea pigs. At a concentration of 4% TCC did not exhibit a photoallergic activity in guinea pigs. In rabbit eyes, TCC caused no discernable irritation in the rinsed group and was only mildly irritating in the un-rinsed group. TCC was tested in groups of 80 Sprague Dawley rats per sex in a two year chronic feeding study at dose levels of 0, 25, 75 and 250 mg/kg bw/day. Mean body weight of males at 250 mg/kg bw/day and females at 75 and 250 mg/kg bw/day were slightly reduced compared to controls during most of the study. Anemia was seen in males at 75 and 250 mg/kg bw/day and females at 250 mg/kg bw/day. Blood chemistry analysis showed a slight increase in alkaline phosphatase, blood urea nitrogen, glucose and total bilirubin at various time points for the high-dose males. There was no evidence for dose-related increases in tumor incidence at any site. The long-term effect to rats of TCC was also tested in a three-generation reproduction study. At the highest TCC dose level, the mean number of live pups at birth was lower than controls in the litters for the F0 generation. A similar trend was not observed in the F1 and F2 generation. TCC has been shown to enhance testosterone-induced effects in vitro and to enlarge accessory sex organs in castrated male rats. TCC was negative for the induction of structural and numerical chromosome aberrations in CHO cells. TCC was considered not to be mutagenic with and without metabolic activation in the Ames test (Salmonella typhimurium strains TA98, TA100, TA1535 and TA 1537). ECOTOXICITY STUDIES: TCC has been detected in aquatic ecosystems and the results of toxicity studies indicate a potential risk to the environment. TCC induced systemic toxic effects in nematode Caenorhabditis elegans. TCC was also reproductively toxic to fish at concentrations at or near those that have been measured in surface water. The effects of TCC on mortality, population growth, lifespan, and fecundity were examined in the monogonont rotifer (Brachionus koreanus) using cellular ROS levels, GST enzymatic activity, and gene expression of defensomes. In TCC-exposed B. koreanus, growth retardation and reduced fecundity were observed and were shown to have a potentially deleterious effect on the life cycle of B. koreanus. In addition, time-dependent increases in ROS contents and GST enzymatic activity were shown in response to TCC exposure. In the reproduction test with the New Zealand mudsnail Potamopyrgus antipodarum TCC caused significantly increased embryo numbers at all tested concentrations, except in the group of 0.170 ug/L. TCC inhibited the growth of T. thermophila and the impairment of plasma membrane was observed after 2 hr exposure of TCC. Furthermore, it is noticeable that at environmentally relevant concentrations (1.0 ug/L), TCC can lead to statistically significant DNA damage in Tetrahymena thermophila, while the inhibition of growth and change of cell viability cannot be observed. In the freshwater mudsnail Potamopyrgus antipodarum after four weeks, environmentally relevant TCC concentrations of 1.6 to 10.5 ug/L resulted in significant increases in the number of unshelled embryos, whereas 0.2, 1.6, and 10.5 ug/L exposures significantly increased numbers of shelled embryos. Interactions Triclocarban (TCC) is an antimicrobial agent routinely detected in surface waters that has been hypothesized to interact with the vertebrate endocrine system. The present study examined the effects of TCC alone and in combination with the model endocrine disruptor 17beta-trenbolone (TRB) on fish reproductive function. Adult Pimephales promelas were continuously exposed to either 1 ug TCC/L or 5 ug TCC/L, to 0.5 ug TRB/L, or to a mixture (MIX) of 5 ug TCC/L and 0.5 ug TRB/L for 22 d, and a variety of reproductive and endocrine-related endpoints were examined. Cumulative fecundity was significantly reduced in fathead minnows exposed to TRB, MIX, or 5 ug TCC/L. Exposure to 1 ug TCC/L had no effect on reproduction. In general, both TRB and MIX treatments caused similar physiological effects, evoking significant reductions in female plasma vitellogenin, estradiol, and testosterone, and significant increases in male plasma estradiol. Based on analysis of the ovarian transcriptome, there were potential pathway impacts that were common to both TRB- and TCC-containing treatment groups. In most cases, however, those pathways were more plausibly linked to differences in reproductive status than to androgen-specific functions. Overall, TCC was reproductively toxic to fish at concentrations at or near those that have been measured in surface water. There was little evidence that TCC elicits reproductive toxicity through a specific mode of endocrine or reproductive action, nor that it could augment the androgenic effects of TRB. Nonetheless, the relatively small margin of safety between some measured environmental concentrations and effect concentrations suggests that concern is warranted. Many widely used healthcare products contain antiseptics, whose persistence in aquatic environments, soils, and sediments leads to the contamination of ecosystems and adversely affects wildlife. Recently, the impact not only of high but also low doses of contaminants and mixtures of several chemicals has become a focus of concern. In this study, toxicity tests of the antiseptics triclosan (TCS) and triclocarban (TCC) were performed in an aquatic test medium using the nematode Caenorhabditis elegans. Nominal concentrations of TCS and TCC were tested in separate single-substance toxicity tests (96-hr-exposure), focusing on growth and reproduction endpoints. Median effective concentrations (EC50s) from the single-substance tests were subsequently used to set up five different ratios of TCS:TCC mixtures leading to the same toxicity. Six dilutions of each mixture ratio were tested for effon reproduction of C. elegans. In the single-substance tests, TCC was about 30 times more toxic than TCS when considering effects on growth and concerning reproduction, TCC was about 50 times more toxic than TCS. For both substances, the toxic effect on reproduction was more pronounced than the one on growth. Low doses of TCS (1-10 umol/L) stimulated reproduction by up to 301% compared to the control, which might be due to endocrine disruption or other stress-related compensation responses (hormesis). Neither antiseptic stimulated growth. In the mixtures, increasing amounts of TCC inhibited the stimulatory effects of TCS on reproduction. In addition, the interactions of TCS and TCC were antagonistic, such that mixtures displayed lower toxicity than would have been expected when TCS and TCC mixtures adhered to the principle of concentration addition. Effects of chemical mixtures at environmentally relevant concentrations on endocrine systems of aquatic organisms are of concern. Triclocarban (TCC) and inorganic mercury (Hg2+) are ubiquitous in aquatic environments, and are known to interfere with endocrine pathways via different mechanisms of toxic action. However, effects of mixtures of the two pollutants on aquatic organisms and associated molecular mechanisms were unknown. This study examined effects of binary mixtures of TCC and Hg2+ on histopathological and biochemical alteration of reproductive organs in zebrafish (Danio rerio) after 21 d exposure. The results showed that: 1) At concentrations studied, TCC alone caused little effect on hepatic tissues, but it aggravated lesions in liver caused by Hg2+via indirect mechanisms of disturbing homeostasis and altering concentrations of hormones; 2) Histological lesions were more severe in gonads of individuals, especially males, exposed to the binary mixture. Exposure to TCC alone (2.5 or 5 ug/L) (measured concentration 140 or 310 ng/L) or Hg2+ alone (5 ug/L or 10 ug/L (measured concentration 367 or 557 ng/L) slightly retarded development of oocytes, whereas co-exposure to nominal concentrations of 5 ug/L TCC and 10 ug /L Hg2+ promoted maturation of oocytes. In males, maturation of sperm was slightly delayed by exposure to either TCC or Hg2+, while their combinations caused testes to be smaller and sperm to be fewer compared with fish exposed to either of the contaminants individually; 3) Lesions observed in fish exposed to binary mixtures might be due to altered transcription of genes involved in steroidogenesis, such as cyp19a, 3beta-HSD, cyp17, 17beta-HSD and modulated concentrations of testosterone and estradiol in blood plasma. The observed results further support the complexity of toxic responses of fish exposed to lesser concentrations of binary chemical mixtures. Since it is impossible to collect empirical information in controlled studies of all possible combinations of toxicants, the application of omics methods might improve the predictive capabilities of results of single classes of chemicals. Estrogen regulates numerous developmental and physiological processes. Most effects are mediated by estrogen receptors (ERs), which function as ligand-regulated transcription factors. Estrogen also regulates the activity of GPR30, a membrane-associated G protein-coupled receptor. Many different types of environmental contaminants can activate ERs; some can bind GPR30 as well. There is growing concern that exposure to some of these compounds, termed xenoestrogens, is interfering with the behavior and reproductive potential of numerous wildlife species, as well as affecting human health. Here, we investigated how two common, environmentally pervasive chemicals affect the in vivo expression of a known estrogen target gene in the brain of developing zebrafish embryos, aromatase AroB, which converts androgens to estrogens. We confirm that, like estrogen, the well-studied xenoestrogen bisphenol A (BPA, a plastics monomer), induces strong brain-specific overexpression of aromatase. Experiments using ER- and GPR30-selective modulators argue that this induction is largely through nuclear ERs. BPA induces dramatic overexpression of AroB RNA in the same subregions of the developing brain as estrogen. The antibacterial triclocarban (TCC) by itself stimulates AroB expression only slightly, but TCC strongly enhances the overexpression of AroB that is induced by exogenous estrogen. Thus, both BPA and TCC have the potential to elevate levels of aromatase and, thereby, levels of endogenous estrogens in the developing brain. In contrast to estrogen, BPA-induced AroB overexpression was suppressed by TCC. These results indicate that exposures to combinations of certain hormonally active pollutants can have outcomes that are not easily predicted from their individual effects. For more Interactions (Complete) data for Triclocarban (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rabbit dermal >10,000 mg/kg bw LD50 Mouse oral > 5,000 mg/kg bw LD50 Mouse ip 2100 mg/kg bw LD50 Rat oral > 2,000 mg/kg bw |
| References |
[1]. Nanomolar concentration of triclocarban increases the vulnerability of rat thymocytes to oxidative stress. J Toxicol Sci. 2013 Feb;38(1):49-55. [2]. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol. 2014 Sep;34(9):1060-7. [3]. Early life triclocarban exposure during lactation affects neonate rat survival. Reprod Sci. 2015 Jan;22(1):75-89. |
| Additional Infomation |
Triclocarban appears as fine white plates or white powder. (NTP, 1992) Triclocarban is a member of the class of phenylureas that is urea substituted by a 4-chlorophenyl group and a 3,4-dichlorophenyl group at positions 1 and 3 respectively. It has a role as a disinfectant, an antiseptic drug, an antimicrobial agent, an environmental contaminant and a xenobiotic. It is a dichlorobenzene, a member of monochlorobenzenes and a member of phenylureas. It is functionally related to a 1,3-diphenylurea. Triclocarban, with the chemical formula C13H9Cl3N2O is an antibacterial agent that is particularly effective against Gram-positive bacteria such as *Staphylococcus aureus*. It is a bacteriostatic compound that has been found in antibacterial soaps and other personal care products. In 2017, the US FDA prohibited the marketing of over-the-counter (OTC) consumer antiseptic wash products containing triclocarban due to negative health effects such as bacterial resistance or hormonal effects,. Triclocarban is a triclosan analogue with an antibacterial property. Triclocarban exerts its effect by inhibiting the activity of enoyl-(acyl-carrier protein) (ACP) reductase, widely distributed in bacteria, fungi and plants. ACP reductase catalyses the last step in each cycle of fatty acid elongation in the type II fatty acid synthase systems. As a result, this agent interrupts cell membrane synthesis and leads to bacterial growth inhibition. Drug Indication Triclocarban (TCC), or 3,4,4'-trichlorocarbanilide, is an antibacterial agent used in bar and liquid soaps and body washes. Mechanism of Action Triclocarban is a triclosan analog with an antibacterial activity. Triclocarban exerts its effect by inhibiting the activity of _enoyl-(acyl-carrier protein) (ACP) reductase_, which is ubiquitously distributed in bacteria, fungi and various plants. ACP reductase catalyzes the last step in each cycle of fatty acid elongation in the type II fatty acid synthase systems. As a result, this agent interrupts cell membrane synthesis and leads to bacterial growth inhibition. As a carbanilide, /triclocarban/ can be classified according to its antimicrobial mechanism as a membrane active compound. The mode of action can be described as unspecific adsorption to cell membranes, interruption of the function of interstitial proteins and/or loss of the semipermeability of the membrane, with discharge of ions and organic molecules. Bacteriostatic or bactericidal effects occur dependent on the concentration. In its standard application concentrations, triclocarban inhibits primarily the growth of gram-positive bacteria, but also that of gram-negative bacteria. Unlike antibiotics, membrane-active antimicrobial substances such as triclocarban are effective within a short period of time. Therapeutic Uses Antiseptic, disinfectant. /EXPL THER/ The increasing use of the antimicrobial triclocarban in personal care products has resulted in concern regarding environmental pollution. Triclocarban is a potent inhibitor of soluble epoxide hydrolase (sEH). Inhibitors of sEH (sEHIs) are anti-inflammatory, anti-hypertensive and cardio-protective in multiple animal models. However, the in vivo effects anticipated from a sEHI have not been reported for triclocarban. Here we demonstrated the anti-inflammatory effects in vivo of triclocarban in a murine model. Triclocarban was employed in a lipopolysaccharide (LPS)-challenged murine model. Systolic blood pressure, plasma levels of several inflammatory cytokines and chemokine, and metabolomic profile of plasma oxylipins were determined. Triclocarban significantly reversed LPS-induced morbid hypotension in a time-dependent manner. Triclocarban significantly repressed the increased release of inflammatory cytokines and chemokine caused by LPS. Furthermore, triclocarban significantly shifted the oxylipin profile in vivo in a time-dependent manner towards resolution of inflammation as expected from a sEHI. These results demonstrated that at the doses used triclocarban is anti-inflammatory in the murine model. This study suggests that triclocarban may provide some benefits in humans in addition to its antimicrobial activities due to its potent inhibition of sEH. It may be a promising starting point for developing new low volume high value applications of triclocarban. However these biological effects also caution against the general over use of triclocarban in personal care products. Pharmacodynamics The antimicrobial mechanism underlying the bacteriostatic and bactericidal effects of triclocarban is believed to be an unspecific adsorption to cell membranes and interruption of their function. As a result, the growth of gram-positive as well as gram-negative bacteria is inhibited. |
Solubility Data
| Solubility (In Vitro) |
DMSO : 63100 mg/mL ( 199.63~316.88 mM ) Ethanol : ~5 mg/mL |
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (7.92 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (7.92 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 3: ≥ 2.5 mg/mL (7.92 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 4: 10% DMSO+40% PEG300+5% Tween-80+45% Saline:2.5 mg/mL (7.92 mM) (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1688 mL | 15.8438 mL | 31.6877 mL | |
| 5 mM | 0.6338 mL | 3.1688 mL | 6.3375 mL | |
| 10 mM | 0.3169 mL | 1.5844 mL | 3.1688 mL |